By PharmaCompass
2023-02-02
Impressions: 2616
In 2022, the world finally began to emerge out of Covid-related restrictions. Though 2020 and 2021 saw several travel-related curbs, there was no let up in the speed at which generic active pharmaceutical ingredient (API) manufacturers were submitting Drug Master Files (DMFs) to the US Food and Drug Administration (FDA). In the four years between 2018 and 2021, the number of Type II DMF submissions (i.e. submissions for drug substance, drug substance intermediate, and material used in their preparation, or drug product) remained steady at over 630 per year.
In 2022, the DMF submissions rose at an impressive pace of 12.1 percent. A total of 1,024 DMFs (Types II, III, IV and V) were submitted in 2022, as opposed to 913 in 2021.
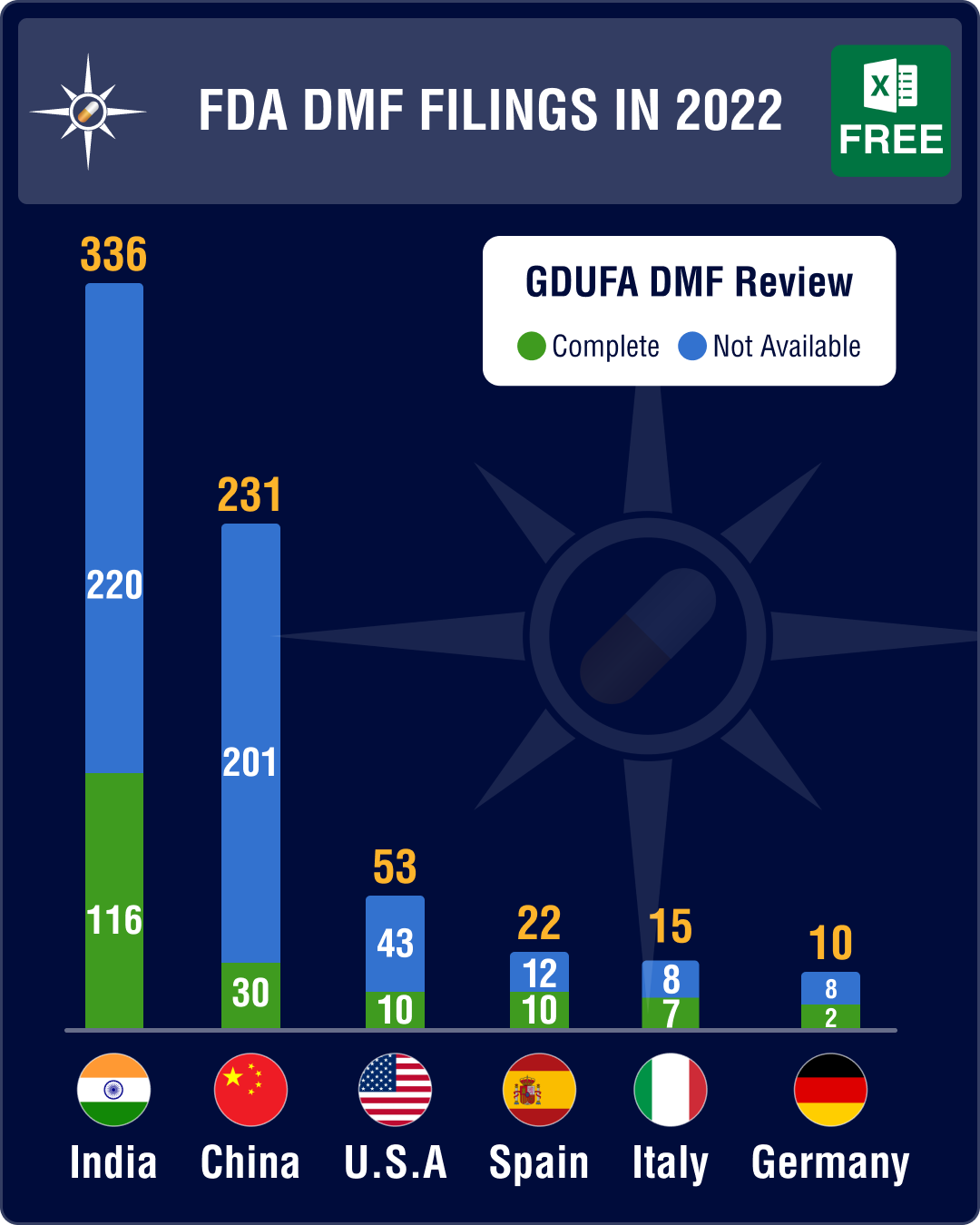
Last year, Type II DMF submissions increased by 7.2 percent — a total of 715 Type II DMFs were submitted in 2022 as opposed to 667 in 2021. However, of the 715 DMFs filed with the FDA, only 190 (26 percent) had their reviews completed.
View FDA DMF Filings in 2022 (Power BI Dashboard, Free Excel Available)
DMF submissions from India dip 10.6%, China’s filings increase 45%
As always, DMFs filed from India and China were significantly higher than DMFs from other countries. However, the year saw DMF submissions from India drop by 10.6 percent. Overall DMF submission from India stood at 336, as opposed to 376 in 2021. In comparison, China’s DMF filings increased by 45 percent to 231 in 2022, as opposed to 159 in 2021.
Interestingly, FDA had issued 31 Form 483s in 2022 out of which 15 were issued to Indian drugmakers. Out of 15, seven Form 483s were issued to manufacturing units belonging to four Indian API manufacturers — Lupin, Aurobindo Pharma, Torrent and Biocon Biologics.
While DMFs are submissions to FDA that may be used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products, a Form 483 is a notification sent by the FDA to a drugmaker regarding objectionable conditions at a facility. A Form 483 is issued at the conclusion of an inspection, when an investigator finds regulatory violations and/or non-compliance of good manufacturing practices. January 2023 saw a spate of Form 483s issued to Indian drugmakers, raising concerns within the pharma industry.
View FDA DMF Filings in 2022 (Power BI Dashboard, Free Excel Available)
India’s Metrochem API tops DMF submissions
India’s Metrochem API led the list of companies with the highest number of DMF submissions at 19. MSN Labs stood second with 16 submissions.
Other top Indian companies were Aurobindo Pharma (15), Biophore India (15), Hetero Group (11) and Cipla (10), followed by two Chinese companies — BrightGene Bio-Medical Technology and Changzhou Pharmaceutical Factory — with 9 submissions each.
The maximum number of DMF filings were for elagolix sodium (11), empagliflozin (eight), tofacitinib citrate (six), revefenacin (six), apixaban (five), brivaracetam (five), cannabidiol (five), followed by mirabegron (five) and tafamidis (five).
A total of 79 DMFs were filed for the first time for 64 products, of which 28 had their GDUFA (Generic Drug User Fee Amendments) review completed. These include revefenacin (six), migalastat hydrochloride (three), cupric sulfate (three), ivosidenib (two), upadacitinib (one) and voxelotor (one). Revefenacin is losing its marketing exclusivity this year.
View FDA DMF Filings in 2022 (Power BI Dashboard, Free Excel Available)
Our view
In our last (H1 2022) review of DMF submissions, we had mentioned that less than half of the 350 Type II DMFs filed by the FDA had been reviewed, as the user fee program had not been reauthorized.
FDA published the New GDUFA III fee structure at the eleventh hour — on September 30, 2022. GDUFA III includes several enhancements to the abbreviated new drug application (ANDA) assessment process to maximize the efficiency and utility of each assessment cycle. These enhancements aim to reduce the number of assessment cycles and facilitate timely access to safe, effective, high-quality and affordable generics.
With the GDUFA III, the US government has made clear its intent to lower generic drug prices. In future, more drugs are likely to go off-patent and we are likely to see the number of DMF submissions rise even further.
In the second quarter of 2022, FDA resumed unannounced inspections in India. The regulator has also stepped up onsite inspections. FDA wants cheaper drugs, but not at the cost of quality. There are no short-cuts for generic and bulk drug manufacturers wanting to export to the US.
(This article has been updated to accurately reflect the information on Metrochem API and MSN Labs.)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA DMF Filings in 2022 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








