DMF filings rise 4.5% in Q3 2025; China holds lead, India records 20% growth in submissions
The
third quarter (Q3) of 2025 witnessed a steady rise in Drug Master File (DMF) submissions to the
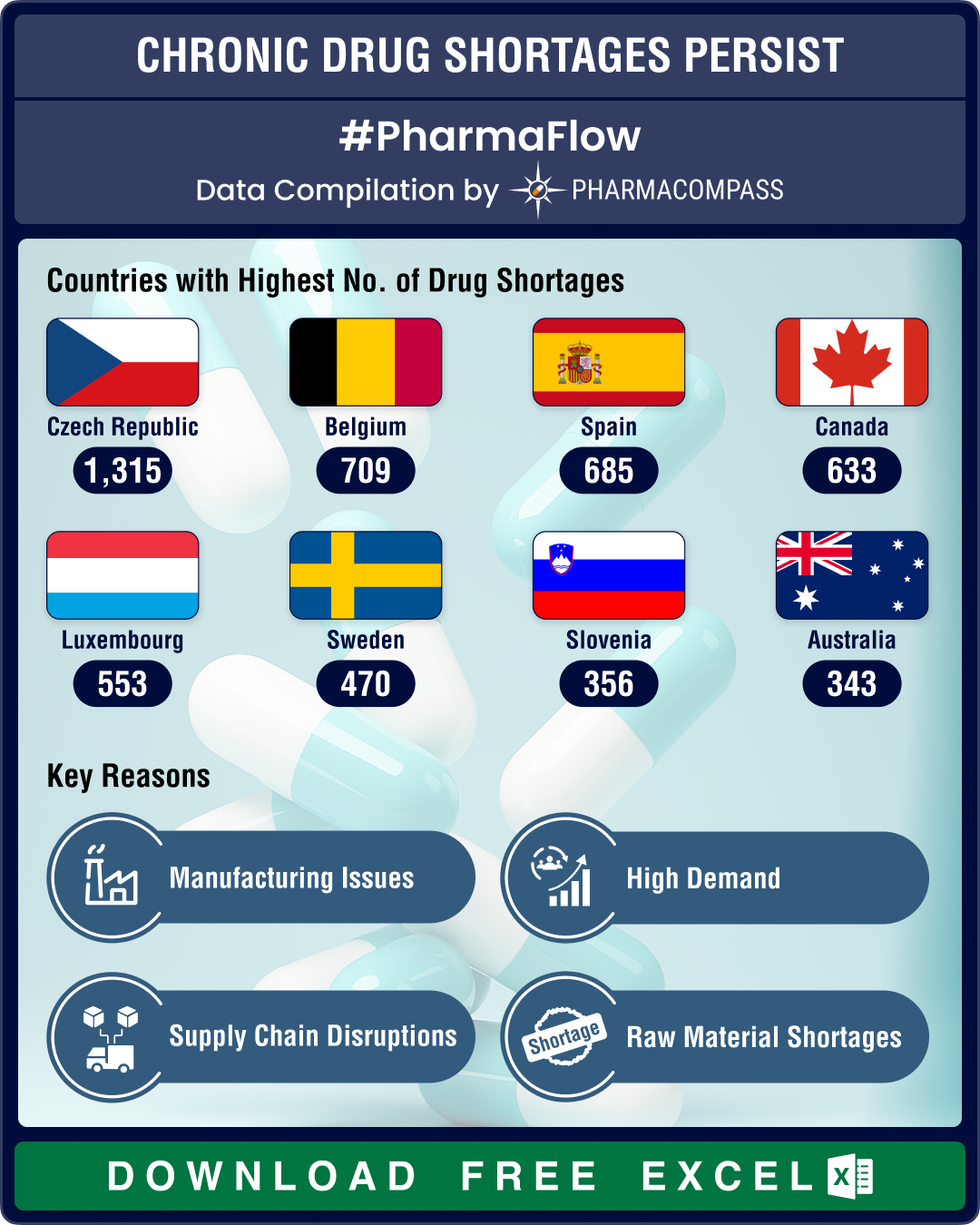
US drug shortages reduce 16% YoY in Q1 2025; CNS drugs, antimicrobials face highest scarcities
The pharmaceutical industry in the United States continued to
grapple with drug shortages during th
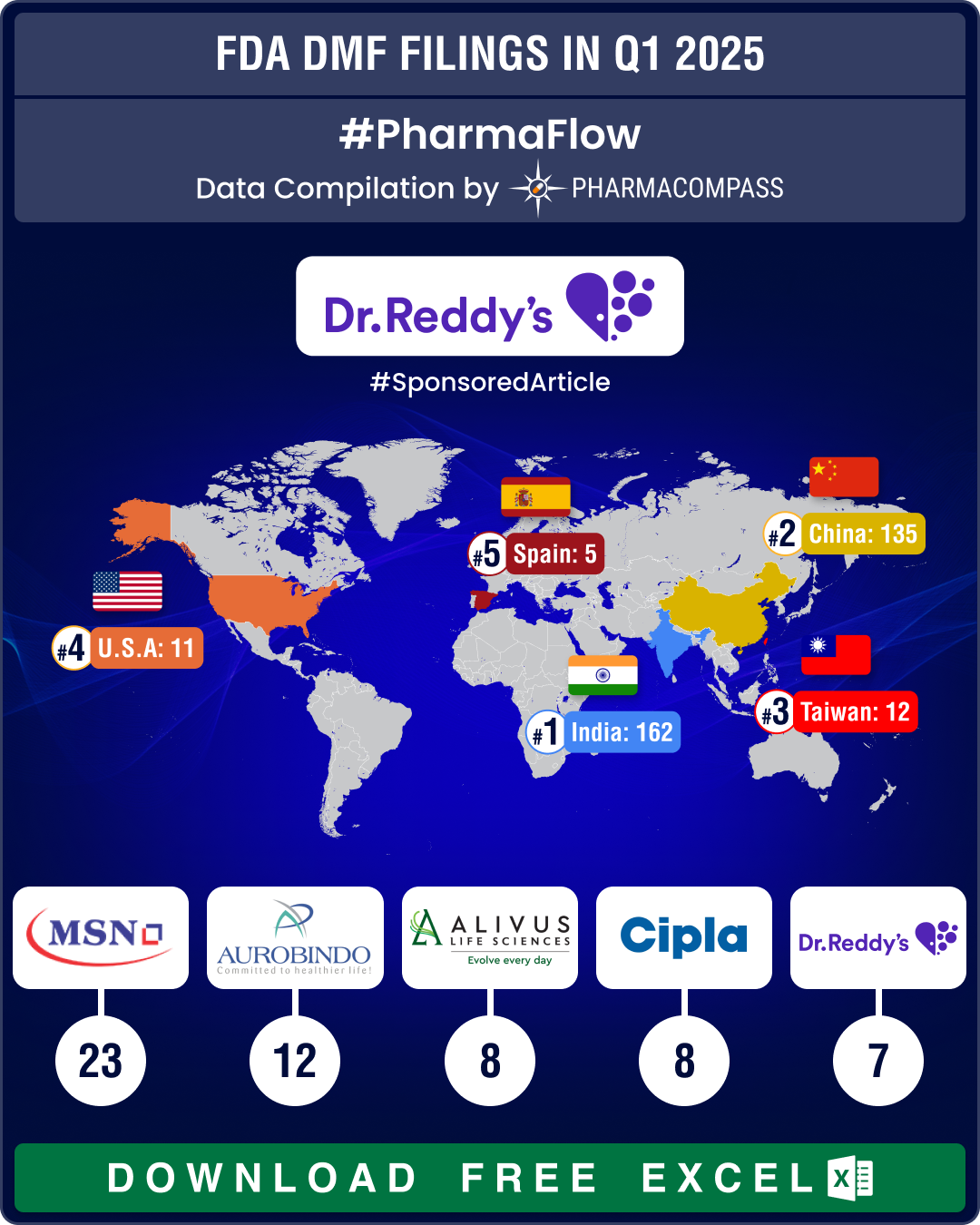
DMF filings surge 44% in Q1 2025; India tops list with 51% rise in year-on-year submissions
The first quarter (Q1) of 2025 witnessed an impressive surge in Drug Master File (DMF) submissions t
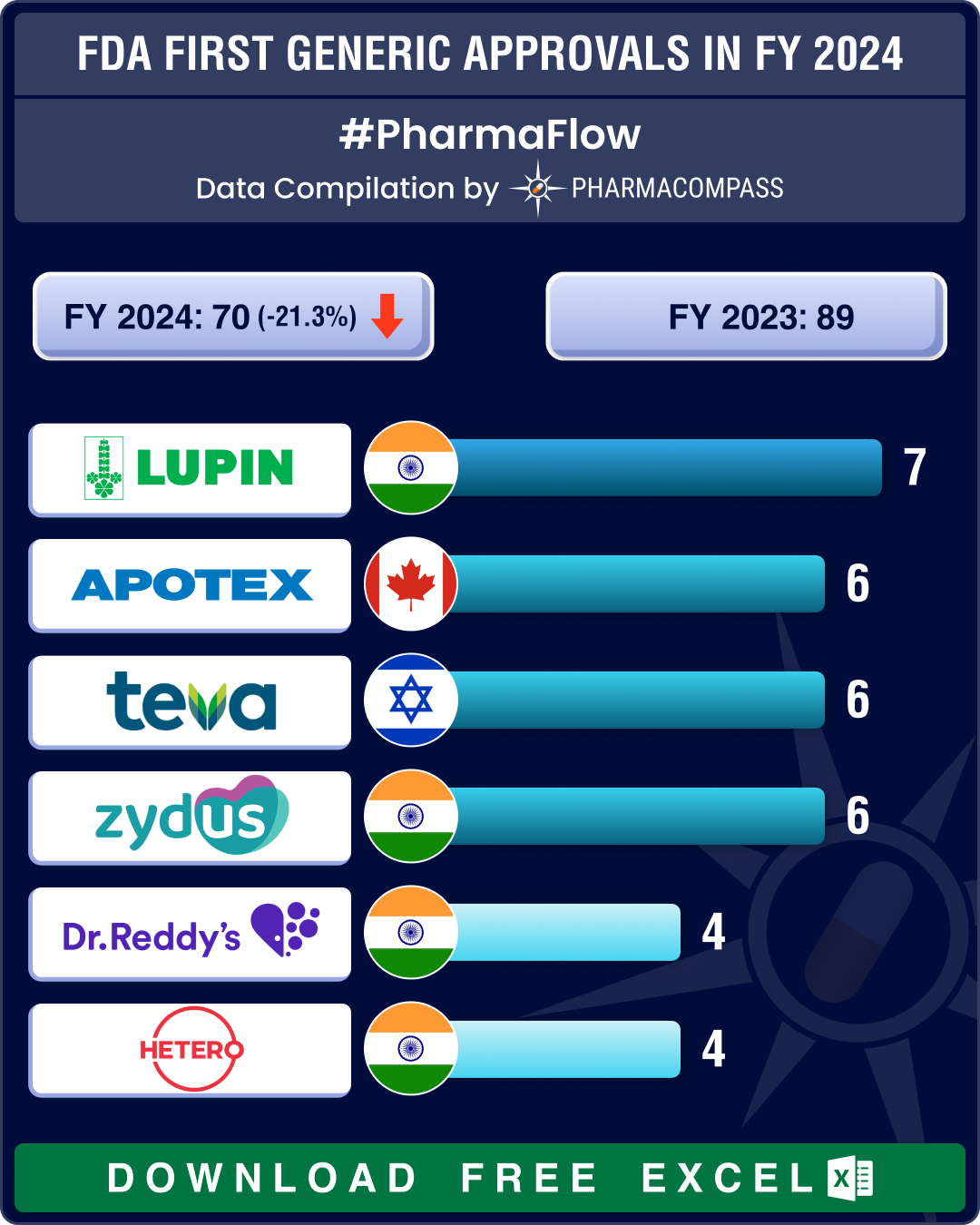
FDA’s first generic approvals slump 21% in 2024; Novartis’ top seller Entresto, cancer blockbuster Tasigna lead 2024 patent cliff
A watershed moment in the journey of a drug is when it transitions from being a patented, high‐
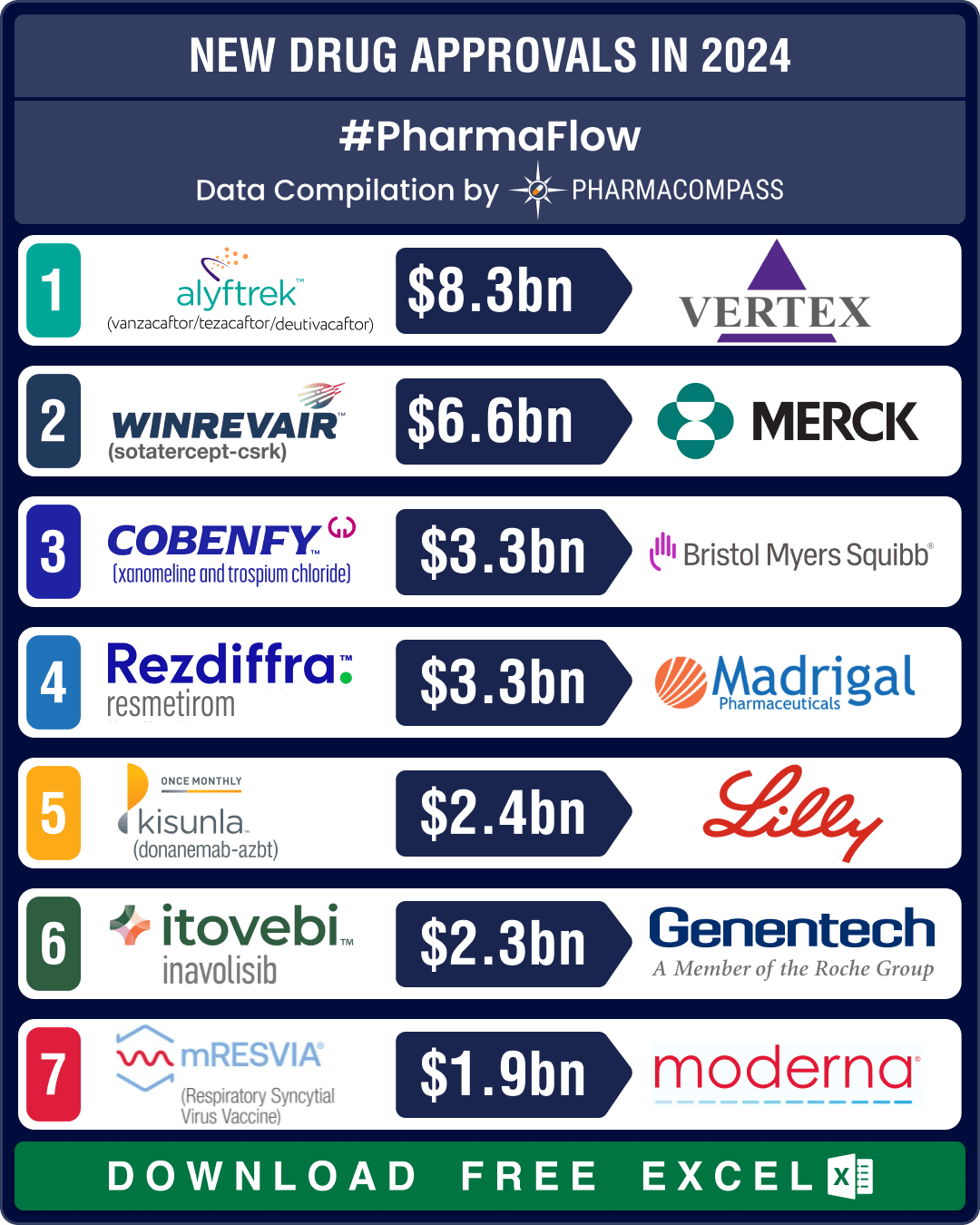
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
CDMO Activity Tracker: Bora, PolPharma make acquisitions; Evonik, EUROAPI, Porton announce technological expansions
The contract development and
manufacturing organization (CDMO) space continued to grow at an impres
Chinese FDA-registered generic facilities gain steam, India maintains lead with 396 facilities
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect
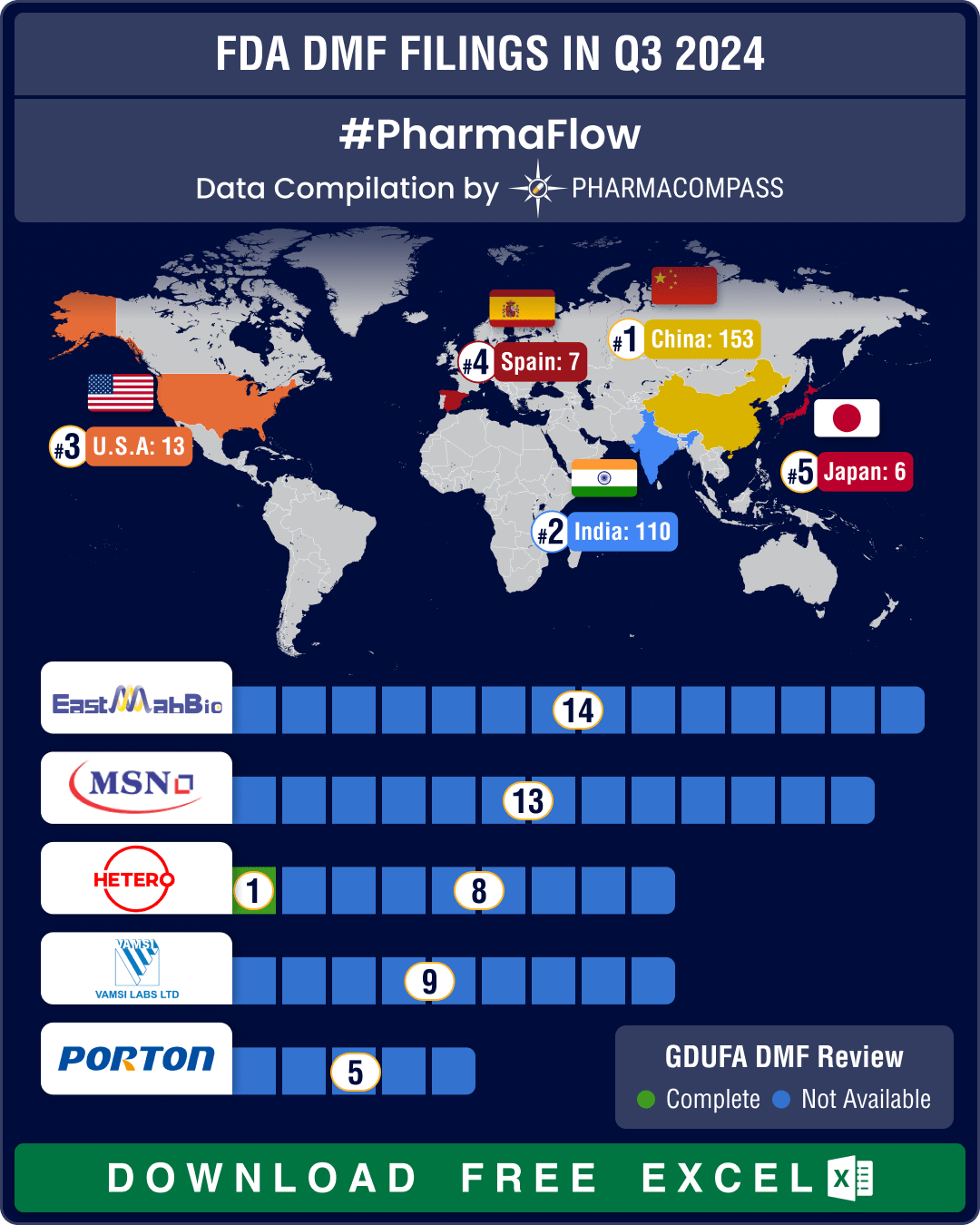
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica
CDMO Activity Tracker: Novo’s parent buys Catalent for US$ 16.5 bn; Fujifilm, Merck KGaA, Axplora expand capabilities
During the first half (H1) of 2024, the global contract development and manufacturing organization (


 Market Place
Market Place Sourcing Support
Sourcing Support