By PharmaCompass
2023-11-23
Impressions: 4097
Both the US and Europe have been grappling with acute drug shortages since the start of the pandemic.
Despite efforts, drug shortages are persisting and affecting a range of medicines, such as those used to manage body weight, cancer drugs, drugs that treat attention deficit/hyperactivity disorder (ADHD), antibiotics and cardiac medications. The latest to join this list is the recently approved therapy for respiratory syncytial virus (RSV) from AstraZeneca and Sanofi — Beyfortus (nirsevimab).
Over the last five to six years, shortages of over 25 new molecules have been reported in the US each year. By June 2023, a total of 160 new drug shortages had been documented, but only 51 of them had been resolved. The US Food and Drug Administration (FDA) has been working closely with drug manufacturers to overcome this ongoing problem.
The scenario is much the same in Europe where there has been a 20-fold increase in drug shortages over the last 20 years. Given the continued shortages of antibiotics, the European Medicines Agency (EMA) is undertaking steps to oversee critical tasks, such as monitoring and reporting drug shortages to EU countries, coordinating responses, and managing the supply and demand of medicinal products. Additionally, EMA plans to collaborate with the Health Emergency Preparedness and Response Authority (HERA) to ensure the availability of antibiotics for respiratory infections through collaborations with concerned drugmakers.
View Our Interactive Dashboard on Drug Shortages in H2 2023 (Free Excel Available)
Astra-Sanofi’s RSV med faces overwhelming demand; CDC prioritizes availability
According to the Centers for Disease Control and Prevention (CDC), about 58,000 to 80,000 children under the age of five years are hospitalized in the US each year and up to 300 die from RSV. These numbers explain the higher-than-expected demand for AstraZeneca and Sanofi’s jointly developed RSV drug Beyfortus (nirsevimab), which has resulted in its shortage. Beyfortus is a monoclonal antibody and the first preventive option approved in July to protect infants in the US.Moreover, CDC has recommended that available doses of Beyfortus be prioritized for infants at the highest risk of severe RSV disease. The organization has also expedited the release of over 77,000 additional doses of Beyfortus and is in close contact with manufacturers to step up supplies through late 2023 and early 2024. In an interview with Reuters, AstraZeneca’s CEO Pascal Soriot said it is prioritizing the US market for additional doses of Beyfortus.
View Our Interactive Dashboard on Drug Shortages in H2 2023 (Free Excel Available)
Rising demand, production delays lead to shortages of diabetes, obesity meds
The US and several European countries such as Belgium, Germany and Hungary continue to face shortages of diabetes and obesity drugs. Resulting largely from increased demand and production delays, these drugs include Novo Nordisk’s semaglutide (sold under brand names Wegovy, Ozempic and Rybelsus) and liraglutide (Victoza and Saxenda) and Eli Lilly’s tirzepatide (Mounjaro) and dulaglutide (Trulicity).
So acute is the problem that Belgium has limited the use of Novo’s semaglutide and GLP-1 drugs to individuals with type 2 diabetes and weight-loss patients meeting specific body mass index criteria. And Germany is considering restricting the export of Novo’s Ozempic.
With Goldman Sachs estimating obesity drugs to attain a market size of US$ 100 billion by 2030, both Novo and Lilly are busy expanding their manufacturing capacities. Novo is investing over Danish kroner 42 billion (US$ 6 billion) to enhance production in Kalundborg, Denmark, and Lilly will be building its first plant in western Germany for € 2.3 billion (US$ 2.5 billion) to meet the soaring demand for its diabetes and weight loss drugs. Novo has also brought Thermo Fisher onboard as a contract manufacturer for its weight loss drug Wegovy.
View Our Interactive Dashboard on Drug Shortages in H2 2023 (Free Excel Available)
Shortages of critical cancer drugs persist; FDA steps in to increase supplies
In our last update, we had captured a severe shortage of cancer drugs that was deemed a national emergency by oncologists in the US. FDA's drug shortage database tells us that as of November 18, the shortage of cancer drugs persists in the US. The drugs in short supply include cisplatin, carboplatin, methotrexate, capecitabine, clofarabine, leucovorin calcium, and azacitidine.
As part of its push to alleviate shortages, FDA has partnered with Chinese drugmaker Qilu Pharmaceutical and Canadian company Apotex to temporarily import cisplatin. Also, it has worked closely with manufacturers to boost production capacity.
In September, the White House issued a statement that said its efforts to resolve the shortage of cisplatin have borne fruit, as the US supply of the chemo drug has been restored to nearly 100 percent of the pre-shortage levels. Recently, Accord Healthcare (the US unit of Intas) announced it has restarted the production of cancer drugs cisplatin and methotrexate.
View Our Interactive Dashboard on Drug Shortages in H2 2023 (Free Excel Available)
High demand, manufacturing issues lead to scarcity of ADHD drugs
During 2020 and 2021, the US experienced a notable increase in the diagnosis of ADHD, leading to over 10 percent rise in stimulant prescriptions across diverse age groups. Record-high demand and manufacturing issues have led to a shortage of ADHD drugs, such as Takeda’s Vyvanse (lisdexamfetamine dimesylate) and methylphenidate hydrochloride (a stimulant sold under several brand names, such as Ritalin, Concerta, Methylin etc).
In an attempt to ease the shortages, FDA has approved generics to Takeda’s Vyvanse capsules earlier this summer after its patent expired in August.
The other drugs in short supply include statins, antibiotics, and cardiac drugs. The US has grappled with a surge in demand for liquid Amoxicillin, essential for the treatment of infections in children. Manufacturers like Teva, Sandoz, and Alembic have made the API available on an ‘allocation basis’ to meet the demand.
Similarly, cardiovascular drugs like adenosine and lidocaine (used to treat arrhythmias) and medications to lower cholesterol, such as rosuvastatin and atorvastatin, are also experiencing shortages.
View Our Interactive Dashboard on Drug Shortages in H2 2023 (Free Excel Available)
Our view
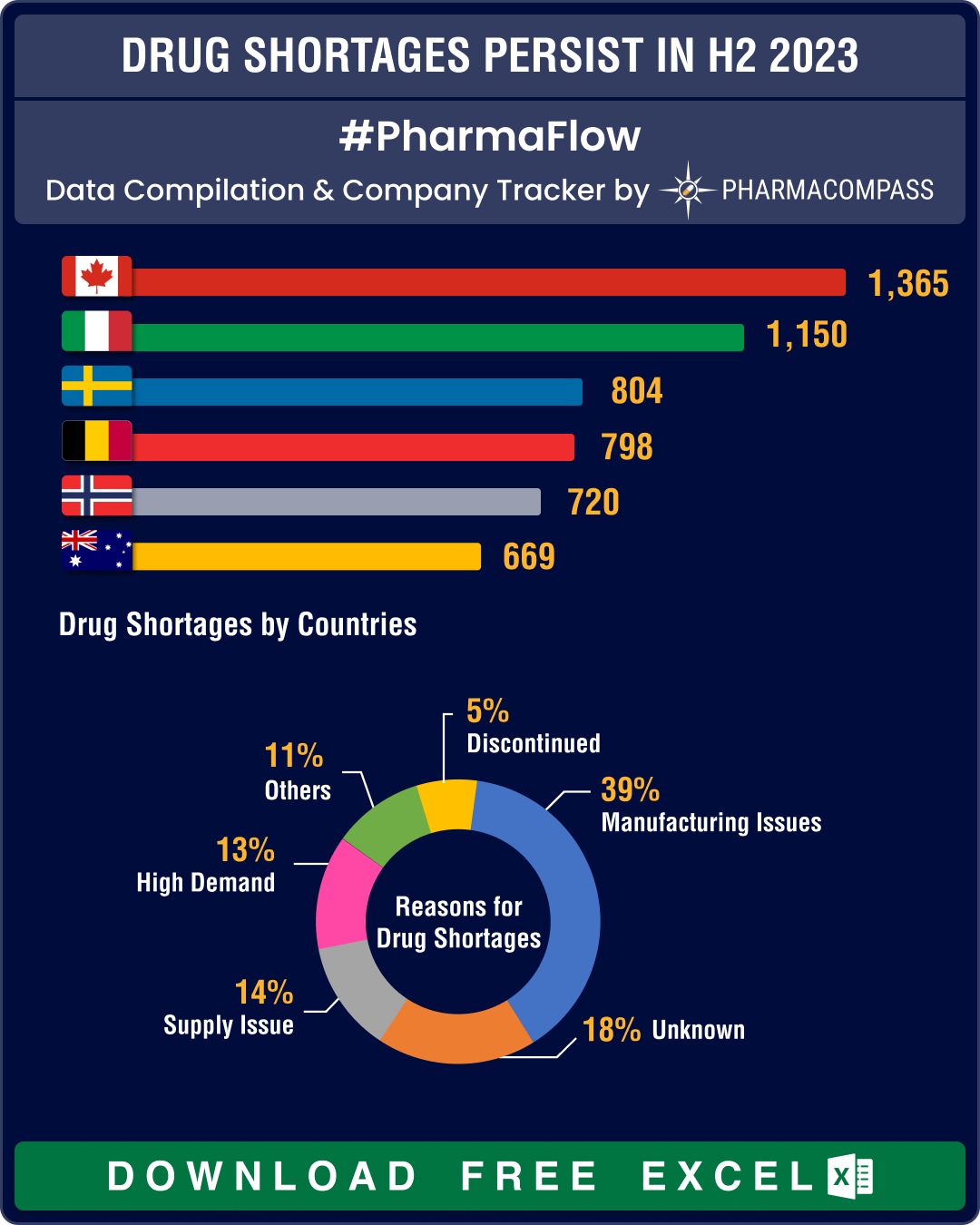
The geopolitical and economic situations coupled with thinning of margins in the generics industry are continuing to impact the biopharma industry. However, our analysis tells us that governments are able to address drug shortages. For instance, in the US, the list of drug facing shortages had 90 drugs on it during January to mid-June 2023. This number has come down to 68 in FDA’s latest update. Similarly, Canada has brought down shortages from 454 to 441 during the same period.
However, in Europe, drug shortages have been worsening over the last six months, owing to several factors such as increased demand, inflation and geopolitical unrest. In Italy, drug shortages have increased from 423 to 541, in Norway from 320 to 370, and in Spain from 295 to 342.
Let’s hope the situation improves in 2024.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Drug shortages persist in H2 2023: Which countries are most affected? by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








