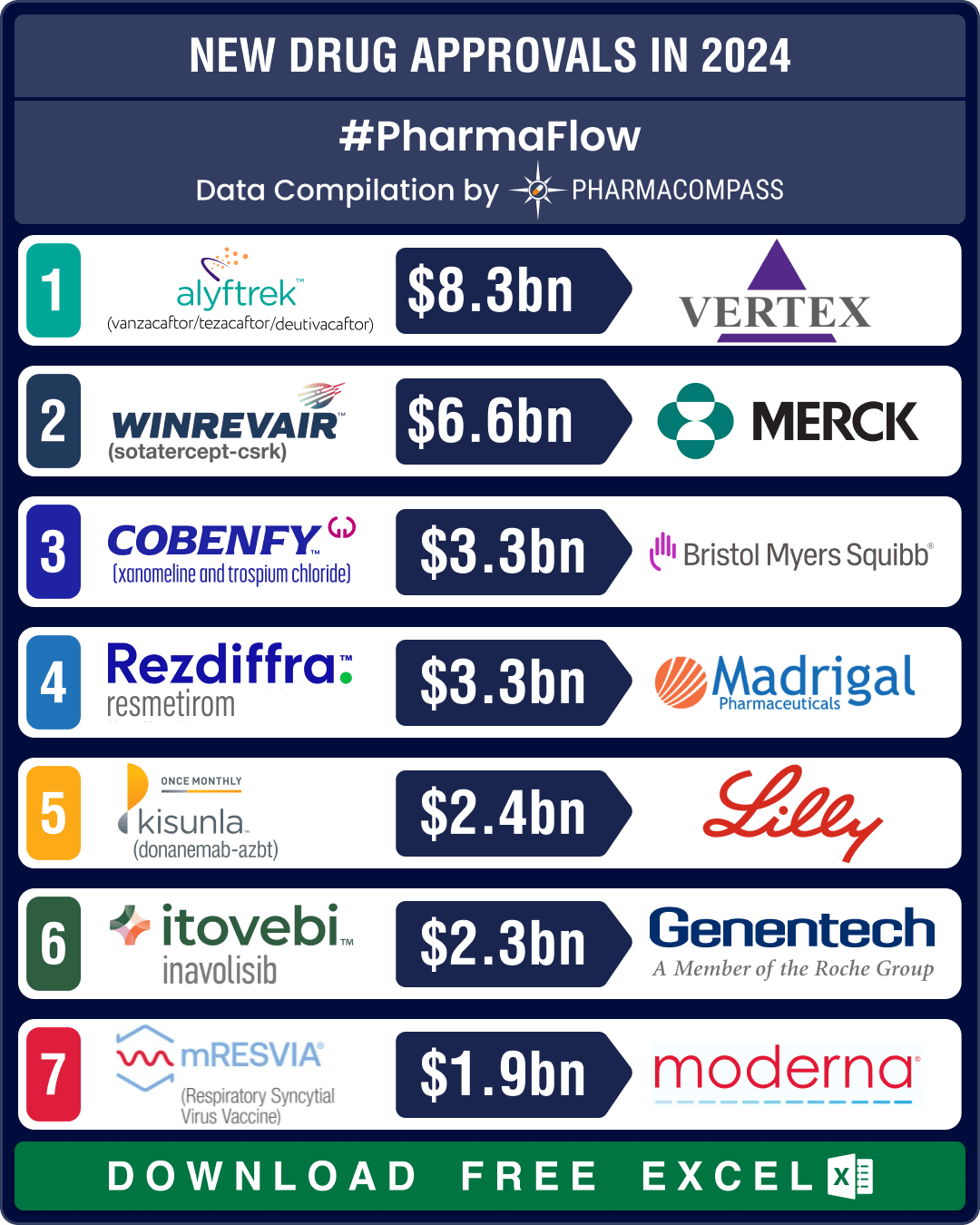
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
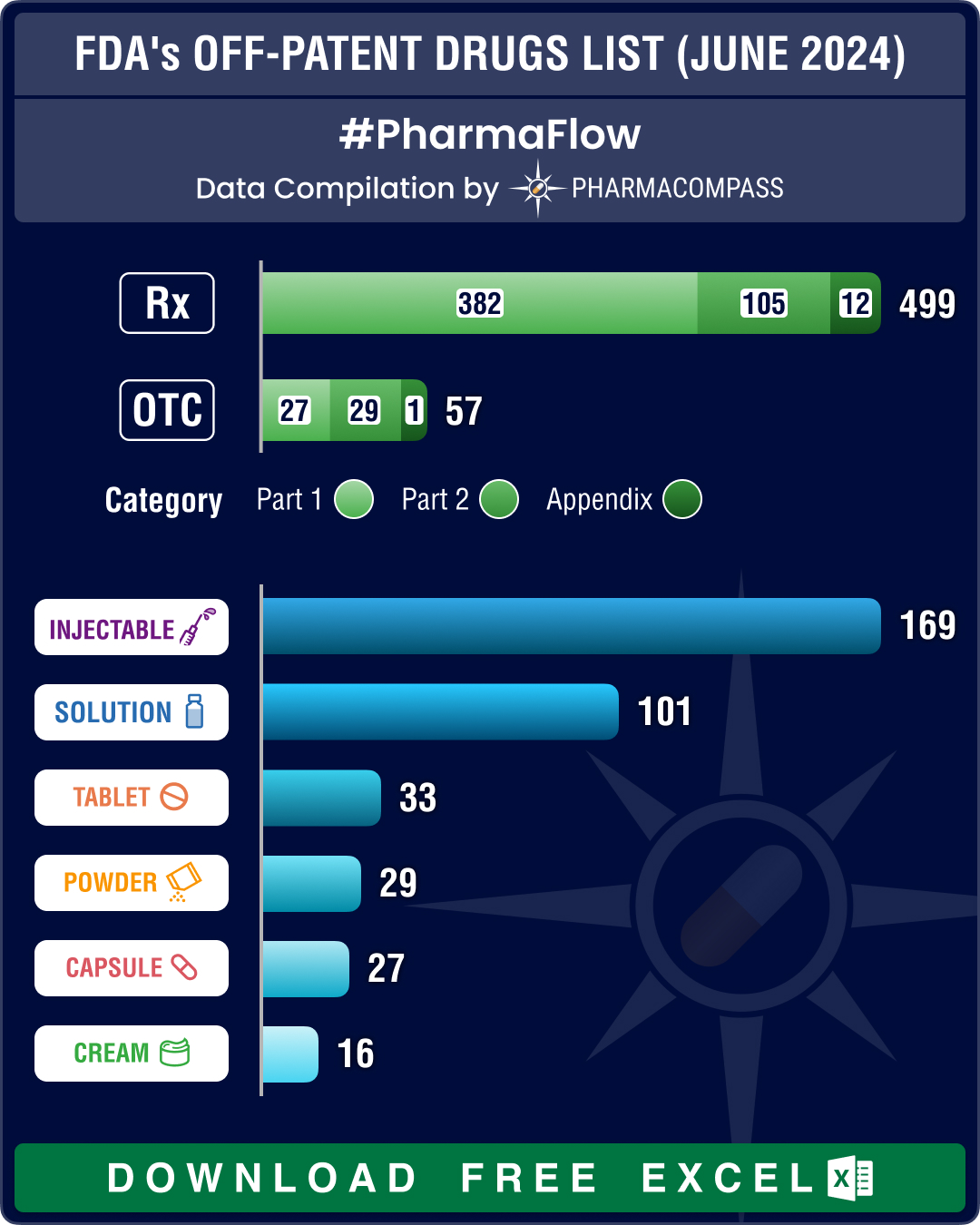
Medical Breakthroughs in 2024: Alzheimer’s, schizophrenia, COPD, MASH see pathbreaking treatments
This year has seen pivotal advancements in medical innovation. The US Food and Drug Administration (
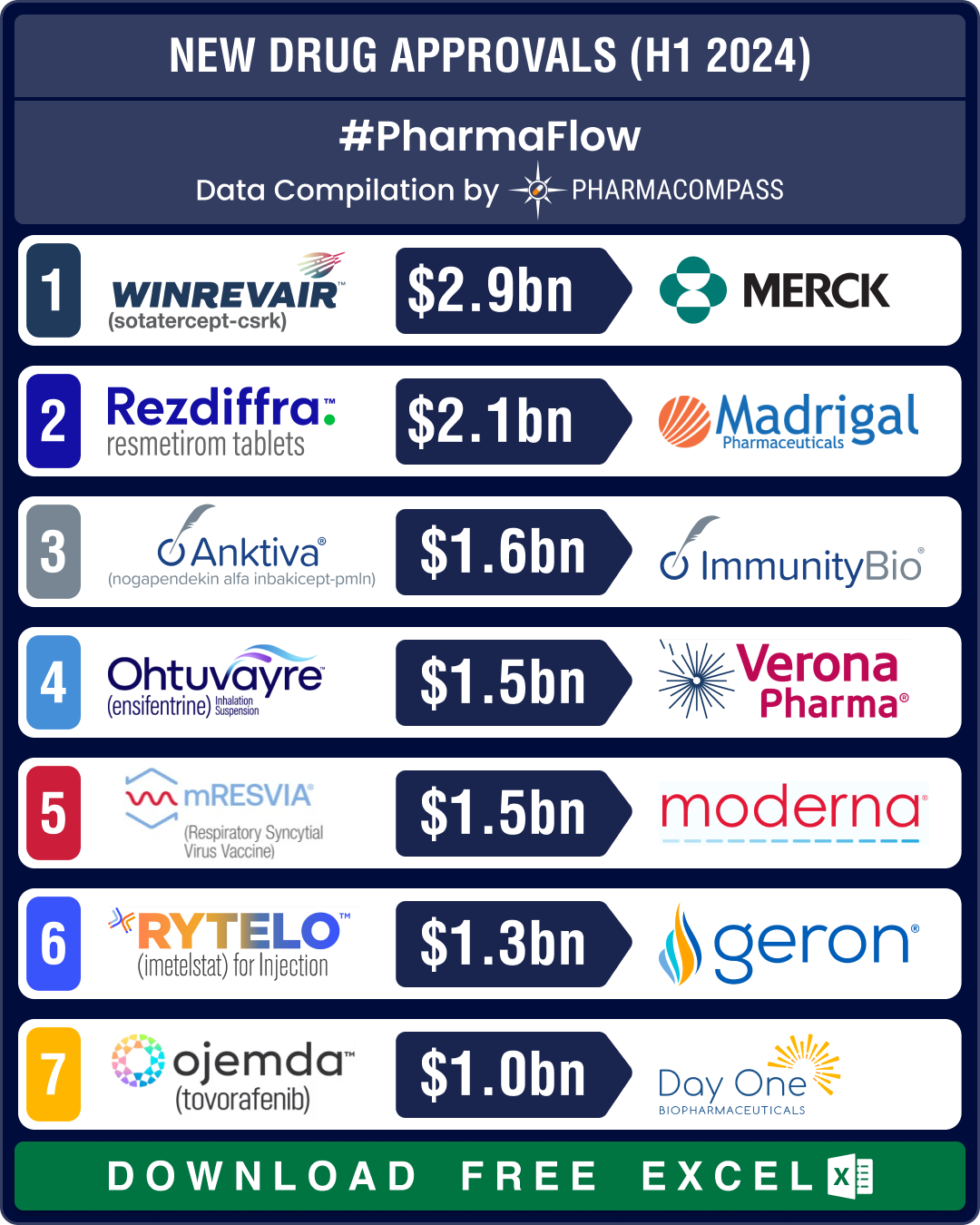
FDA approvals slump 19% in H1 2024; NASH, COPD, PAH get new treatment options
The first half of 2024 saw a significant slowdown in approvals of new drugs and biologics by the US


 Market Place
Market Place Sourcing Support
Sourcing Support