Last week, the United States and the European Union (EU) finally announced that they will be able to utilize each other’s good manufacturing practice (GMP) inspections of pharmaceutical manufacturing facilities.
The deal is expected to enable the US Food and Drug Administration (FDA) and the EU to avoid duplication of drug inspections, lower inspection costs and enable regulators to devote more resources to other parts of the world where there may be greater risk.
While the idea sounds excellent, as it will reduce the inspection burden experienced by organizations as well, industry watchers have long been aware of the shortcomings of pharmaceutical quality inspections.
This week, PharmaCompass looks at why such a reliance on each others’ regulatory inspections may actually be problematic by quoting multiple cases where different conclusions were drawn by the EU and US regulators on the same manufacturing site.
Depending on other regulators ‘is problematic’
The outcomes of regulatory inspections are highly variable and inconsistent as they depend significantly on the capabilities of the investigators, the area under review during the inspection and the state of the facility’s operation at the time when it is inspected.
FDA Law Blog has stated “the notion of having a foreign inspectorate perform drug inspections on FDA’s behalf, when the inspections performed by FDA’s own investigators are already so inconsistent, is problematic at best.”
FDA’s own Office of Pharmaceutical Quality has accepted that inspection findings “have not been a reliable predictor of the state of quality.”
Discrepancies in regulators’ conclusions
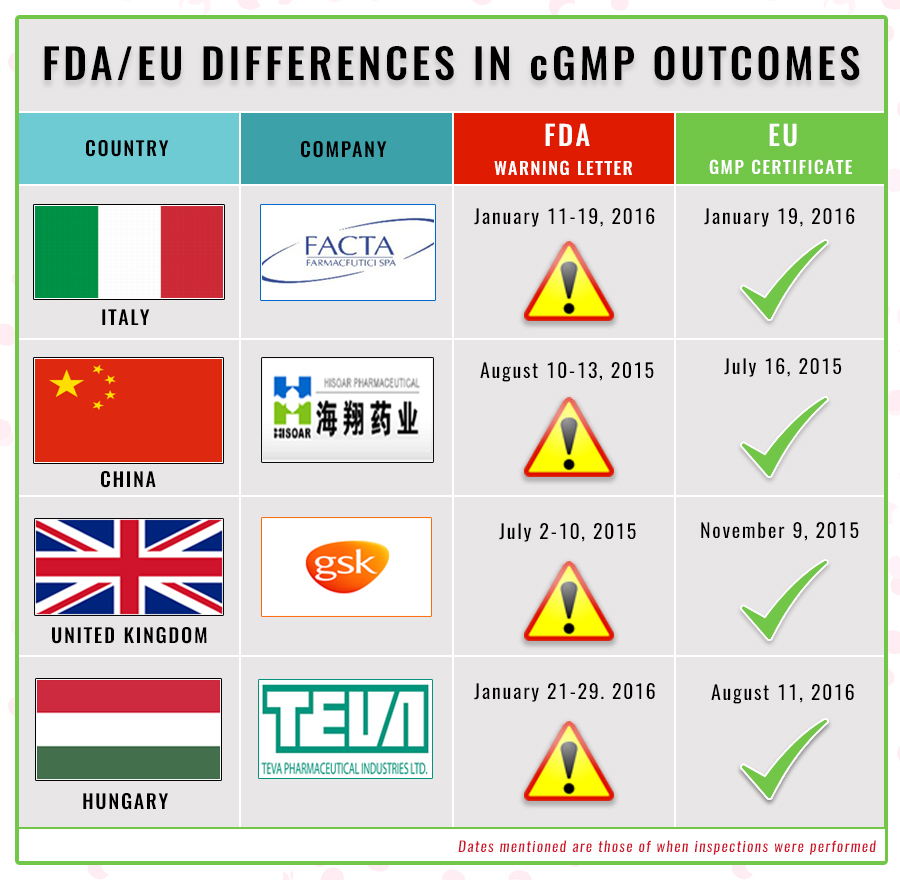
This week, PharmaCompass looked at recent inspections where, within a short period of time, the FDA and EU regulators reached drastically different conclusions about the cGMP standards at the same facility.
A little over a month ago, we had covered the warning letter issued by the FDA to ACS Dobfar’s Italian drug manufacturing facility — FACTA Farmaceutici SpA.
During a January 2016 inspection, FDA investigators uncovered data-integrity violations where for multiple lots of sterile drug product, the original data showed failing results, while the final data reportedly showed passing results.
The company was found storing original data in an “unofficial” and uncontrolled electronic spreadsheet on a shared computer network drive. The analyst told investigators that original data was first recorded in the “unofficial” spreadsheet and later transcribed to an “official” form.
Investigators also observed many copies of uncontrolled blank and partially-completed cGMP forms and also documented that employees at FACTA used paper shredders to destroy critical laboratory and production records.
During an inspection performed exactly at the same time, FACTA’s EU GMP certification was renewed by the Italian regulators.
Another case in point is that of GSK’s facility in the United Kingdom, where the FDA documented “findings of penicillin in non-penicillin manufacturing areas approximately 69 times in 2012, 72 times in 2013, 30 times in 2014, and 16 times through July 7, 2015.”
FDA investigators concluded that the facility and its controls to prevent contamination of non-penicillin drugs with penicillin were wholly inadequate. However, an inspection by the UK regulator approved the site four months later.
Something similar happened at Zhejiang Hisoar in China. Here, FDA investigators discovered a lack of basic laboratory controls. Investigators noted: “When you encountered suspect and out-of-specification (OOS) results, you retested samples until you obtained desirable results.” However, German inspectors who had visited the site three weeks prior to the FDA inspection found the site to be in compliance.
Our analysis
uncovered multiple instances where recent assessments of the same site had
divergent conclusions drawn by the FDA and EU. These are tabulated in the graphic below:
|
FAILED |
APPROVED |
|||||
|
Country |
Company |
Regulator |
Inspection Dates |
Regulator |
Inspection Dates |
Time Gap |
|
Italy |
Jan 11-19, 2016 |
Jan 19, 2016 |
0 days |
|||
|
China |
Aug 10-13, 2015 |
Jul 16, 2015 |
4 weeks |
|||
|
United Kingdom |
Jul 2-10, 2015 |
Nov 9, 2015 |
4 months |
|||
|
United Kingdom |
Oct 5-13, 2015 |
May 11, 2015 |
5 months |
|||
|
Spain |
May 2-6, 2016 |
Nov 4, 2015 |
6 months |
|||
|
Hungary |
Jan 21-29. 2016 |
Aug 11, 2016 |
7 months |
|||
|
Czech Republic |
Oct 12-16, 2015 |
Feb 20, 2015 |
8 months |
|||
|
United States |
Feb 26, 2016 |
May 29, 2015 |
9 months |
|||
|
India |
Dec 7-15, 2015 |
Oct 27, 2016 |
10 months |
|||
|
Italy |
May 21-29, 2015 |
May 2, 2016 |
11 months |
|||
Our view
The inconsistent outcome of inspections make us support FDA Law Blog’s view that the first order of business for the agency, prior to implementing the mutual recognition practice, should be to rectify “these significant lacunae in FDA’s inspectional responsibilities”.
There are new FDA initiatives to address these problems, such as those on Quality Metrics, which will be voluntary until 2018, and the New Inspection Protocol Project (NIPP), which is under development.
The NIPP is expected to provide a more quality-focused, semi-quantitative approach with streamlined and structured inspection reports. The NIPP protocols utilize expert investigator questions and assessment approaches. The NIPP is expected to increase the quality focus of investigator assessments, so that facilities and behaviors found to exceed basic compliance can be recognized as such.
Accelerating these initiatives needs to become a priority, because establishing global pharmaceutical quality standards with inconsistent assessments is not good for the industry.
In fact, they stifle continuous improvement initiatives.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







