By PharmaCompass
2024-02-01
Impressions: 3766
In 2022, when the US Food and Drug Administration (FDA) was reeling under the impact of the pandemic, new drug approvals by the agency dropped by 26 percent. But last year, FDA’s new drug approvals rebounded by an impressive 49 percent, with the Center for Drug Evaluation and Research (CDER) approving 55 new drugs in 2023. Of them, 36 percent were considered first-in-class, while small molecules made up for 62 percent of the total drugs approved (i.e. 34). FDA’s Center for Biologics Evaluation and Research (CBER) okayed 19 biologics in 2023 compared to eight in the previous year.
The first half of 2023 saw the debut of vaccines for the all-too-common respiratory syncytial virus (RSV). Among the other notable approvals in H1 was Biogen and Eisai’s Alzheimer’s drug Leqembi (lecanemab). Out of the total 55 drug approvals, 29 came in H2 2023. This includes Vertex Pharmaceuticals and CRISPR Therapeutics’ Casgevy that relies on the Nobel Prize-winning CRISPR gene-editing technology. Casgevy has been approved as a treatment for sickle-cell disease (SCD) and β-thalassemia.
While FDA witnessed a sharp rise in approvals in 2023, many other drug regulators didn’t. The European Medicines Agency (EMA) granted marketing authorization to 32 novel drugs in 2023, a fall from 33 in 2022. Similarly, Health Canada’s approvals in 2023 decreased to 38, compared to 45 in the previous year.
As usual, oncology topped the list of drug approvals by therapeutic area, at 39 (as opposed to 35 in 2022). Rare diseases was the second most popular therapeutic area for drug approvals. With drugmakers clearly paying heed to the unmet needs of patients suffering from rare diseases, this therapeutic area sprinted from a 9 percent share and the fourth position among new approvals in 2022 to an impressive 34 percent share in 2023. A quarter of the new drug approvals were in infectious diseases, followed by immunology (19 percent) and neurology (7 percent).
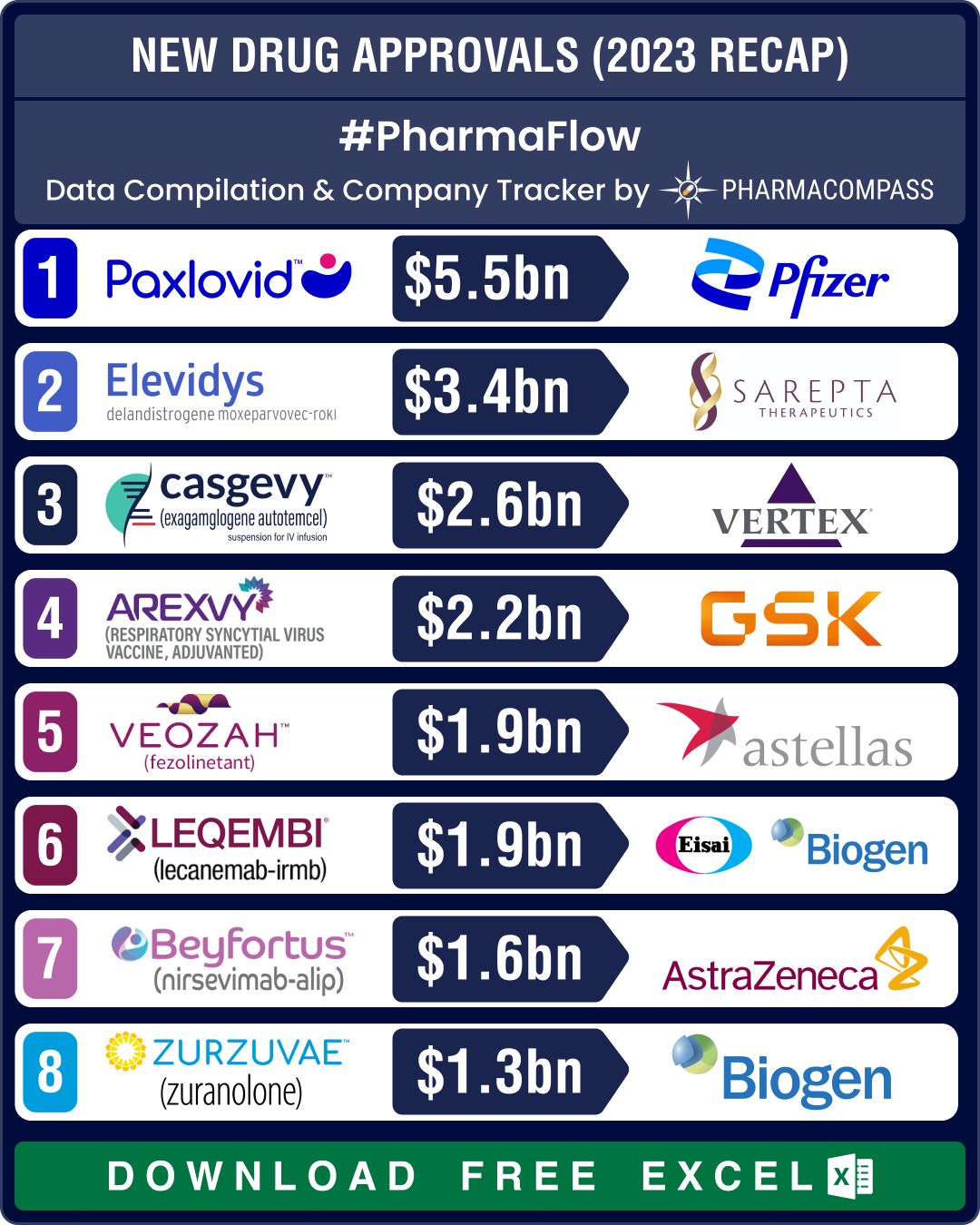
View New Drug Approvals in 2023 with Estimated Sales (Free Excel Available)
Casgevy, postpartum depression drug Zurzuvae emerge as potential blockbusters
Gene therapy Casgevy, postpartum depression (PPD) med Zurzuvae, blood cancer med Elrexfio and ulcerative colitis drug Velsipity were some of the prominent approvals of 2023.
Britain’s Medicines and Healthcare products Regulatory Agency was the first to okay Casgevy in November as a cure for SCD and β-thalassemia. Soon, the FDA approved it for SCD. In January this year, the American agency also approved it for transfusion-dependent β-thalassemia (TDT). Analysts estimate Casgevy to generate US$ 2.6 billion in peak sales, says Nature.
Biogen and Sage’s PPD therapy Zurzuvae became the first and only FDA-approved pill for the condition that can be life-threatening for both the mother and the child. Global sales of Zurzuvae are forecast to hit US$ 1.28 billion by 2028.
In August, Pfizer’s Elrexfio (elranatamab) became the first “off-the-shelf” (ready-to-use) therapy in the US for multiple myeloma. The drug provides an option for patients with hard to treat or relapsed blood cancer and is estimated to bring in US$ 861 million in peak sales by 2028, says Nature.
Pfizer also bagged another significant approval in October — its drug Velsipity (etrasimod) was greenlit by the FDA to treat adults with ulcerative colitis, an inflammatory bowel disease. Peak revenue for Velsipity is expected to come in at US$ 825 million, as per Evaluate.
View New Drug Approvals in 2023 with Estimated Sales (Free Excel Available)
Astra’s Truqap, GSK’s Ojjaara among top cancer therapies given FDA nod in H2
In November, FDA approved AstraZeneca’s Truqap (capivasertib) in combination with the Anglo-Swedish drugmaker’s Faslodex (fulvestrant) for treating adult patients with hormone receptor (HR)-positive, HER2-negative locally advanced or metastatic breast cancer with one or more biomarker alterations. Evaluate Pharma forecasts peak Truqap sales to come in at about US$ 690 million.
In September, FDA approved GSK’s Ojjaara (momelotinib) as the first and only treatment for myelofibrosis with anemia. Nearly all myelofibrosis patients are estimated to develop anemia over the course of the disease. Ojjaara is taken orally once a day.
Other notable oncology treatments okayed by FDA in H2 2023 include Daiichi’s Vanflyta (quizartinib) in July to treat an aggressive blood cancer known as acute myeloid leukemia (AML). In August, FDA approved Janssen’s bispecific antibody Talvey (talquetamab-tgvs) for difficult-to-treat blood cancer. The agency approved two cancer therapies in November — BMS’ Augtyro (repotrectinib) for ROS1-positive non-small cell lung cancer (NSCLC) and Takeda’s targeted oral therapy Fruzaqla (fruquintinib) for adult patients with metastatic colorectal cancer (mCRC).
View New Drug Approvals in 2023 with Estimated Sales (Free Excel Available)
Rare disease drugs Santhera-Catalyst’s Agamree, Novo’s Rivfloza bag approval in H2
In October, FDA approved Santhera Pharmaceuticals and Catalyst Pharma’s Agamree (vamorolone), an oral suspension treatment for Duchenne muscular dystrophy (DMD) in patients two years of age and older. This makes it the first drug fully approved in both the US and Europe for the muscle degeneration disorder. Agamree acts in a manner similar to other steroids, which are the standard of care for the inherited rare disease. However, it causes fewer side effects.
FDA also okayed Novo Nordisk’s once-a-month injection Rivfloza (nedosiran) in October to treat a rare genetic condition — primary hyperoxaluria type 1 (PH1) — that causes recurring kidney stones.
In November, the agency approved Takeda’s Adzynma (ADAMTS13, recombinant-krhn) as the first treatment for both adult and pediatric patients with congenital thrombotic thrombocytopenic purpura (cTTP), a rare genetic blood disorder.
Other noteworthy FDA approvals in H2 2023 for rare blood diseases include Novartis’ Fabhalta and bluebird bio's Lyfgenia. Fabhalta is the first oral monotherapy for the treatment of adults with paroxysmal nocturnal hemoglobinuria, a rare disease that causes symptoms such as hemolytic anemia, hemoglobinuria (excretion of hemoglobin in the urine), fatigue, shortness of breath etc. Lyfgenia is the first cell-based gene therapy for the treatment of SCD in patients 12 years and older. Similarly, another rare disease drug — Regeneron’s Veopoz — bagged FDA approval in August last. Veopoz treats CHAPLE disease, an ultra-rare disease in which patients have severe gastrointestinal problems.
View New Drug Approvals in 2023 with Estimated Sales (Free Excel Available)
Our view
After a lull in 2022, new drug approvals have finally gathered momentum. The good news is that this year, several pathbreaking drugs are coming up for approval, such as Madrigal Pharmaceuticals’ resmetirom (the first treatment for NASH with liver fibrosis), Merck’s sotatercept (a treatment for pulmonary arterial hypertension), Lilly’s donanemab for Alzheimer’s disease and Karuna Therapeutics’ drug to treat schizophrenia. Let’s hope 2024 turns out to be an even bigger year for new drug approvals.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : New Drug Approvals (2023 RECAP) by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







