By PharmaCompass
2024-07-25
Impressions: 2546
The first half of 2024 saw a significant slowdown in approvals of new drugs and biologics by the US Food and Drug Administration (FDA) compared to the same period last year.
FDA’s Center for Drug Evaluation and Research (CDER) approved 21 drugs in H1 2024, reflecting a 19 percent decrease from the 26 approvals granted in H1 2023. Of them, 81 percent (17) were first-in-class drugs (therapies that use a new and unique mechanism of action), while small molecules made up for 67 percent (14) of the total drugs approved.
Similarly, the Center for Biologics Evaluation and Research (CBER) granted approvals to only eight biologics, as compared to 10 in H1 2023.
Health Canada also saw a drop in drug approvals as only 10 drugs were okayed in H1 2024, as opposed to 13 approvals in H1 2023.
The European Medicines Agency (EMA) saw a marginal rise in drug authorizations at 15 for H1 2024 as compared to 14 approvals in H1 2023. Interestingly, the EMA also saw a surge in pending decisions (applications under review) — from two in H1 2023 to 14 in H1 2024.
View New Drug Approvals in H1 2024 with Estimated Sales (Free Excel Available)
Merck, Madrigal, Verona bag approvals for breakthrough meds; Lilly’s donanemab okayed
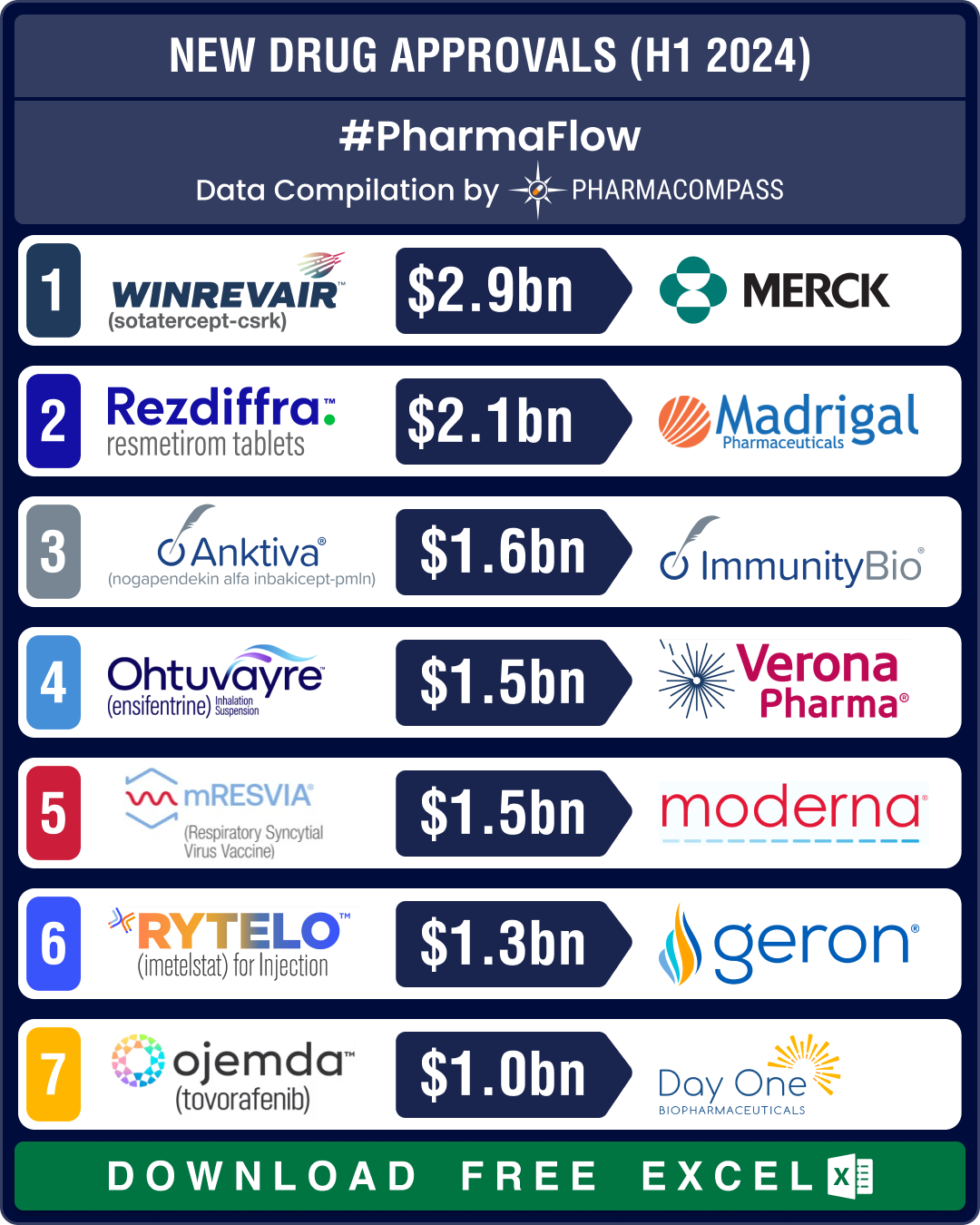
The first half saw some closely watched drugs win regulatory approvals. FDA approved a breakthrough therapy from Merck — Winrevair (sotatercept) — that treats adults with hypertension caused by the constriction of arteries in the lungs, known as pulmonary arterial hypertension (PAH).
Merck had acquired Winrevair through its US$ 11.5 billion acquisition of Acceleron Pharma in 2021. The therapy is set to generate nearly US$ 3 billion in global peak sales by 2028.
Another breakthrough therapy approved in H1 2024 is Madrigal’s Rezdiffra (resmetirom), the first FDA-approved treatment for adults with the common fatty liver disease — nonalcoholic steatohepatitis (NASH). Rezdiffra is expected to touch sales of US$ 2.1 billion by 2028.
The agency also approved the first maintenance treatment for chronic obstructive pulmonary disease (COPD) in over 20 years — Verona’s Ohtuvayre. The drug has a novel mechanism of action and is the first inhaled maintenance treatment for COPD. Approved in June by the FDA, Ohtuvayre is forecast to bring in global sales of US$ 1.5 billion by 2030.
The approval of Eli Lilly’s donanemab was surprisingly delayed, and finally came through on July 2 after an FDA advisory committee voted unanimously in favor of its benefits outweighing its risks. To be sold as Kinsula, the Alzheimer's drug is estimated to bring in US$ 2.2 billion in sales by 2028.
Across the pond, EMA approved Novo Nordisk’s weekly insulin injection Awiqli (insulin icodec). The replacement insulin in Awiqli acts in the same way as the body’s own insulin and helps glucose enter cells from the blood. Meanwhile, FDA rejected this once-a-week insulin earlier this month and has requested information related to the manufacturing process.
View New Drug Approvals in H1 2024 with Estimated Sales (Free Excel Available)
ImmunityBio, Geron, Day One win approvals for their oncology drugs
In what marks the first approval for ImmunityBio, FDA greenlit Anktiva (nogapendekin alfa inbakicept-pmln) as part of a combination therapy to treat a type of bladder cancer. Anktiva is a next-generation immunotherapy that creates long-term immunity by activating the so-called natural killer (NK) cells and T-cells. It will compete with Merck’s Keytruda. Anktiva’s yearly sales by 2030 are expected to be around US$ 1.7 billion.
BeiGene’s PD-1 blocker Tevimbra (tislelizumab) got the go-ahead from the FDA as the treatment for adult patients with unresectable or metastatic esophageal squamous cell carcinoma (ESCC) after prior systemic chemotherapy that did not include a PD-(L)1 inhibitor. Tevimbra’s 2028 global sales are forecast to bring in US$ 1.6 billion.
FDA signed off on Geron’s Rytelo (imetelstat) for treating transfusion-dependent anemia in patients with low- to intermediate-risk myelodysplastic syndromes (MDS), a group of blood cancers. This was Geron’s maiden approval and Rytelo is expected to bring in US$ 1.3 billion by 2030.
Day One Biopharmaceuticals’ Ojemda (tovorafenib) was granted FDA’s accelerated approval to treat certain types of pediatric brain cancer. This is the first FDA approval of a systemic therapy for treating what is the most common form of childhood brain tumor, including fusions. Ojemda is forecast to bring in US$ 1 billion in sales by 2030.
FDA granted accelerated approval to Amgen’s Imdelltra (tarlatamab-dlle) for adults in advanced stages of small cell lung cancer (SCLC) that has proven to be hard to treat or has worsened despite platinum-based chemotherapy. Imdelltra is expected to bring in annual sales of US$ 975 million by 2030.
View New Drug Approvals in H1 2024 with Estimated Sales (Free Excel Available)
Infectious disease drugs from Basilea, Merck, rare disease med from Ipsen bag approvals
After oncology, infections and infectious diseases, and rare diseases were the two therapeutic areas that saw the second and third most approvals, respectively. FDA approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for injection), an antibiotic for bacterial infections including multidrug-resistant strains.
The US agency also approved Merck’s next-generation vaccine designed to protect adults from pneumococcus bacteria that causes serious illnesses and pneumonia. The jab, known as Capvaxive, helped produce an immune response against all 21 variations (serotypes) of the bacteria that it targeted. These 21 strains account for about 85 percent of invasive pneumococcal disease cases in adults aged 65 and above.
FDA also approved Moderna’s mRESVIA, a messenger RNA-based (mRNA) respiratory syncytial virus (RSV) vaccine, to protect adults aged 60 years and older from lower respiratory tract disease caused by the syncytial virus. This is the first non-Covid mRNA vaccine to be approved in the US.
The agency granted accelerated approval to Ipsen’s Iqirvo (elafibranor) to treat primary biliary cholangitis (PBC), a rare liver disease. This is the first new medicine approved in nearly a decade for the treatment of PBC. Orchard Therapeutics’ Lenmeldy secured FDA approval to become the first gene therapy in the US for a rare pediatric disorder, known as metachromatic leukodystrophy (MLD). The debilitating hereditary disease affects the brain and the nervous system and causes loss of cognitive and motor functions and early death.
View New Drug Approvals in H1 2024 with Estimated Sales (Free Excel Available)
Our view
The increased momentum of drug approvals witnessed after the pandemic appears to have slowed down, but what’s encouraging is the increase in first-in-class therapies, cancer drugs and promising new treatment options for a range of conditions such as PAH, NASH, and COPD.
The second half has already kicked off with the approval of Lilly’s donanemab. And there are several pathbreaking drugs likely to be approved soon, such as Karuna Therapeutics’ schizophrenia drug KarXT and BridgeBio’s heart drug acoramidis. There is every possibility that new drug approvals will spring back up in H2 2024.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : New Drug Approvals by FDA, EMA & Health Canada: H1 2024 Recap by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







