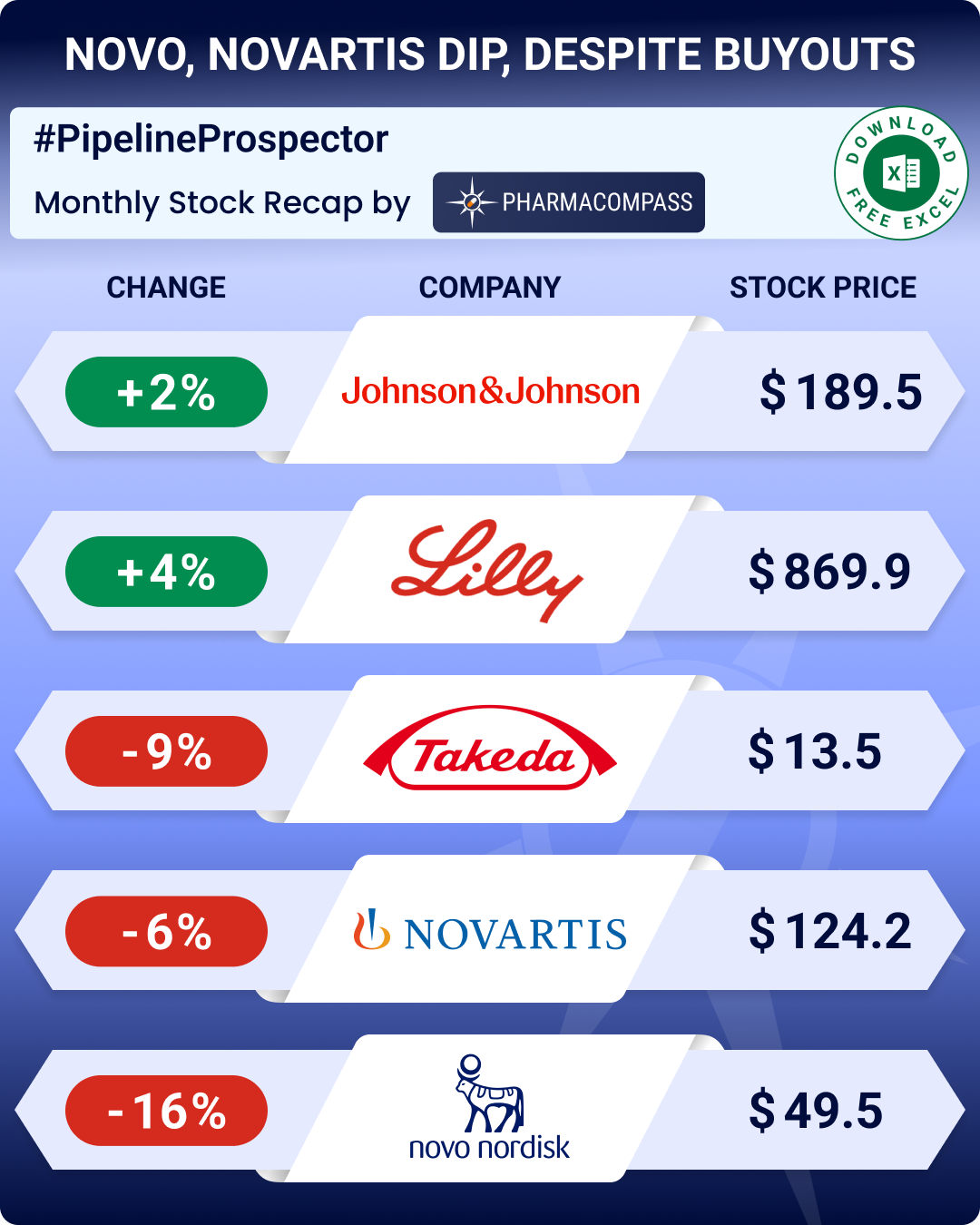
By PharmaCompass
2023-08-24
Impressions: 3814
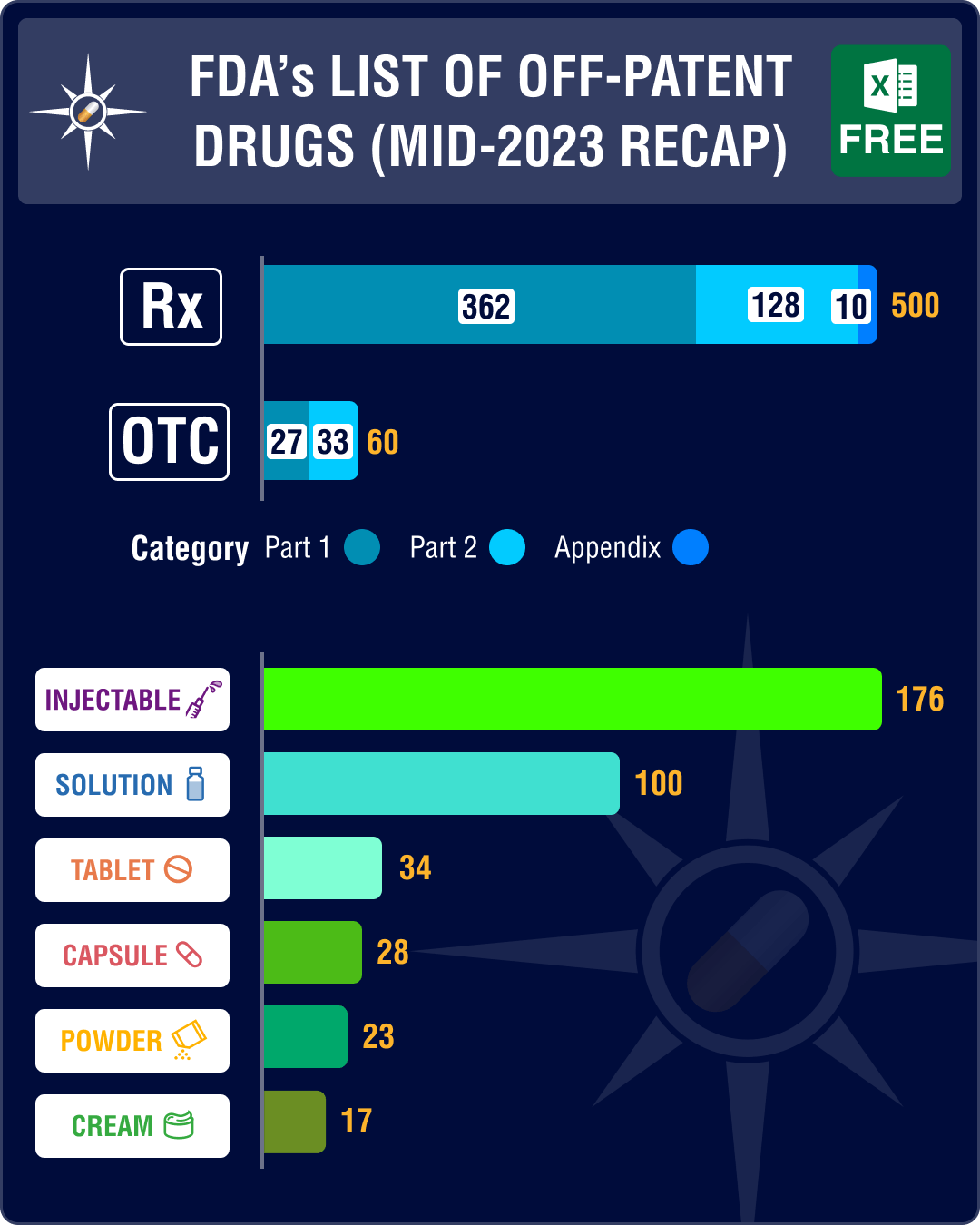
This week, PharmaCompass brings you the key highlights of the US Food and Drug Administration’s June 2023 list of Off-Patent, Off-Exclusivity Drugs without an Approved Generic (“OPOE list”). The OPOE list gets updated every six months.
Since December 2021, FDA has been publishing two versions of the OPOE list, one for prescription (Rx) drug products and the other for over-the-counter (OTC) drug products that are approved and marketed under a new drug approval (NDA).
With this list, the FDA hopes to bolster competitiveness in the generics market. Such updates are a part of FDA’s initiative to improve transparency and encourage the development and submission of abbreviated new drug applications (ANDAs) in markets with little competition.
View FDA's June 2023 list of Off-Patent, Off-Exclusivity Drugs (Free Excel Available)
Sharp rise in new applications; injectables make up for a third of Rx drug products
While the FDA’s December 2022 list of OPOE Drugs with No Approved Generics had four new applications for prescription drugs, the June 2023 list saw a sharp increase — 20 new applications were added during this period. These include glycopyrrolate (a drug used to treat peptic ulcers in adults), atropine sulfate (a drug used for pupil dilation and the treatment of anticholinergic poisoning), bortezomib (used to treat blood plasma cell cancer), cabazitaxel (used to treat prostate cancer), carmustine (chemotherapy drug), citalopram hydrobromide (used to treat depression and anxiety), cocaine hydrochloride (used as local anesthesia for diagnostic procedures and surgeries), and epinephrine (used to treat allergic reaction).Almost one-third of the prescription products – 176 out of 500 – are drug products delivered as injectables while there are 76 entries for oral solid dosage forms (such as tablets, capsules and modified release forms).
The June 2023 list has a total of 60 OTC drug products. These include antiseptic agent chlorhexidine gluconate, non-steroidal anti-inflammatory drug ibuprofen, anti-allergy drug loratadine and painkiller acetaminophen. Out of the 60 OTC drug products on the list, 19 are delivered as oral solid dosage forms (such as tablets, capsules, modified release forms, etc.).
View FDA's June 2023 list of Off-Patent, Off-Exclusivity Drugs (Free Excel Available)
First generic of AstraZeneca’s Symbicort gets launched in US
This year, several large-selling drugs from drugmakers like Johnson & Johnson, Takeda, AstraZeneca, Roche and others are due to face their first generic or biosimilar challengers in the US.
As the name suggests, “first generics” are the first approvals handed by the FDA to manufacturers to market a generic product in the United States. The agency considers first generics to be important to public health, and prioritizes review of these submissions.
Among the more popular drugs, Viatris and Kindeva Drug Delivery launched Breyna (budesonide and formoterol fumarate dihydrate) Inhalation Aerosol this month. This is the first generic version of AstraZeneca’s Symbicort. Breyna is a metered-dose inhaler that relaxes muscles in the airways to improve breathing. It is indicated for certain patients with asthma or chronic obstructive pulmonary disease (COPD).
View FDA's June 2023 list of Off-Patent, Off-Exclusivity Drugs (Free Excel Available)
Our view
Over the last few years, we have noticed FDA’s intent to bring down drug prices. While the sharp rise in new applications for prescription drugs only reinforces that trend, we also need to keep in mind that margins in the generics business have been going down and many large drugmakers are planning to reduce their focus on generics. This leaves more opportunities for smaller players to capture a larger piece of the US market for generics.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics (Mid-2023 Recap) by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”