By PharmaCompass
2022-03-03
Impressions: 4446
In August last year, PharmaCompass had reviewed the key highlights of the US Food and Drug Administration’s June 2021 list of Off-Patent, Off-Exclusivity (OPOE) Drugs with No Approved Generics. This week, we bring you the December 2021 update of the OPOE list.
Since 2017, the FDA has been publishing the OPOE list of drugs without an approved generic. Starting with the December 2021 update, the FDA will publish two versions of the OPOE list — one for prescription drug products and one for over-the-counter (OTC) drug products that are approved and marketed under a new drug approval (NDA).
The OPOE list gets updated every six months. With this list, the FDA hopes to bolster competitiveness in the generics market. Such updates are a part of FDA’s initiative to improve transparency and encourage the development and submission of abbreviated new drug applications (ANDAs) in markets with little competition.
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
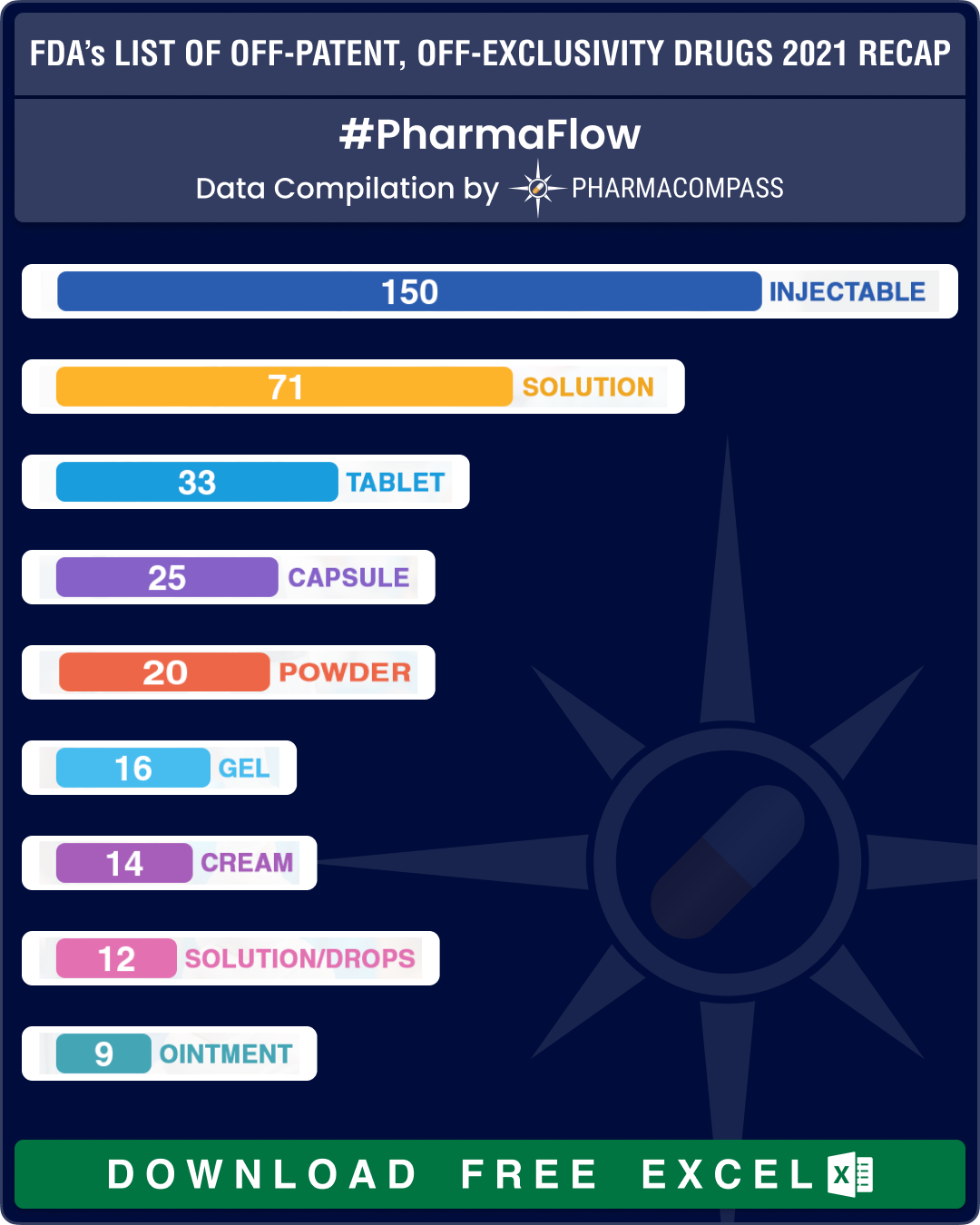
Only 16 new entries figure in latest list
The latest compilation contains 442 entries with 307 being classified as Part I (drug products for which FDA could immediately accept an ANDA without prior discussion), 127 as Part II (drug products for which ANDA development or approval may raise potential legal, regulatory, or scientific issues that should be addressed with the FDA prior to considering submission of an ANDA) and eight products being added to the Appendix. The Appendix identifies NDA drug products that were removed from Part I or Part II of the list because one or more ANDAs referencing such NDA drug products have been approved since the publication of the previous list.
While there were 35 new entries in the June 2021 list, this time there were only 16 new entries. These include antipsychotic medicine amisulpride, ophthalmic medicine atropine sulfate, blood pressure drug chlorthalidone, antihistamines desloratadine and pseudoephedrine, HIV drug emtricitabine, antibiotic eravacycline dihydrochloride and heart drug esmolol hydrochloride.
Almost one-third of the products – 150 out of 442 – continue to be drug products delivered as injectables, while 71 entries on the list are delivered as oral solid dosage forms (such as tablets, capsules, modified release forms, etc.).
A total of 58 OTC drug products figured in the OPOE list. These include disinfectant chlorhexidine gluconate, nonsteroidal anti-inflammatory drug ibuprofen, antihistamine loratadine, antiseptic povidone iodine and analgesic acetaminophen. Out of the 58 OTC drug products on the list, 17 are delivered as oral solid dosage forms (such as tablets, capsules, modified release forms etc.).
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
FDA approved 93 first generics in 2021
In early September, FDA had issued a press statement that said the agency has reached the milestone of approving over 100 generic drug applications with a Competitive Generic Therapy designation. The press statement said this achievement highlights the success of the Competitive Generic Therapy program, “which was designed to encourage the development and marketing of generic drugs for products with little to no competition.”
Clearly, this news was a one-off, as there has been a steady decline in generic approvals in recent years. Last month, the FDA’s Office of Generic Drugs (OGD) 2021 Annual Report pointed out that generic drug approvals continued to fall in 2021. The FDA approved or tentatively approved 776 ANDAs for generic drugs in 2021.
In calendar year 2020, FDA approved or tentatively approved 948 ANDAs for generic drugs, which was down from 1,014 in 2019. The latest figures are part of FDA’s OGD 2021 Annual Report.
However, the good news is that the agency approved 93 “first generics” in 2021, up from 72 in 2020. First generic drugs are drugs that provide access to therapies where no competition previously existed. The FDA prioritizes review of first generic drugs.
“In 2021, even with the unique challenges caused by the ongoing pandemic, OGD continued to innovate and conduct scientific research to keep the FDA generic drug program moving forward,” OGD Director Sally Choe said.
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
Our view
In a 2019 Senate testimony, Janet Woodcock, the then director of the FDA Center for Drug Evaluation and Research, had said: “Since brand-name versions of complex drug products are often higher-priced than many other brand name drugs, efforts to encourage generic competition for complex products also offers outsized potential to increase patient access and lower drug spending.”
Similarly, in January 2020, after Sandoz had announced it would no longer seek approval of generic Advair Diskus, former FDA commissioner Scott Gottlieb had noted: “We must continue efforts to get generic copies of complex drugs to market. There is a large category of brand drugs that are off patent and off exclusivities and should be subject to brisk generic competition, but are not. This is a big opportunity to improve access, lower costs.”
Over the next few years, several biologics are going off-patent that would open up huge opportunities for cost-efficient generic drug manufacturers, particularly those from countries like India and China. For instance, Genentech/Roche’s ranibizumab lost its patent in the US in 2021 and is slated to lose its European patent protection this year. In fact, both the FDA and the European Commission approved a biosimilar of Lucentis — Samsung Bioepis’ Byooviz — last year and other ranibizumab biosimilar candidates are already in advanced phases of clinical trials.
Similarly, Regeneron Pharmaceuticals’ aflibercept will lose its patent protection in the US in 2023 and biologics like Avastin, Humira and Levemir are coming off-patent in 2022 and 2023. According to recent studies, the biosimilars segment is expected to see rapid growth over the next decade — rising from US$ 12 billion in 2020 to at least US$ 36 billion by 2025 at a CAGR of 24 percent.
Surely, 2022 will see more activity in biosimilars, with biotech companies in India and elsewhere gearing up to innovate and establish themselves as key players in the global biosimilars market.
View FDA's List of Off-Patent, Off-Exclusivity Drugs with No Approved Generics
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA's List of Off-Patent, Off-Exclusivity Drugs by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







