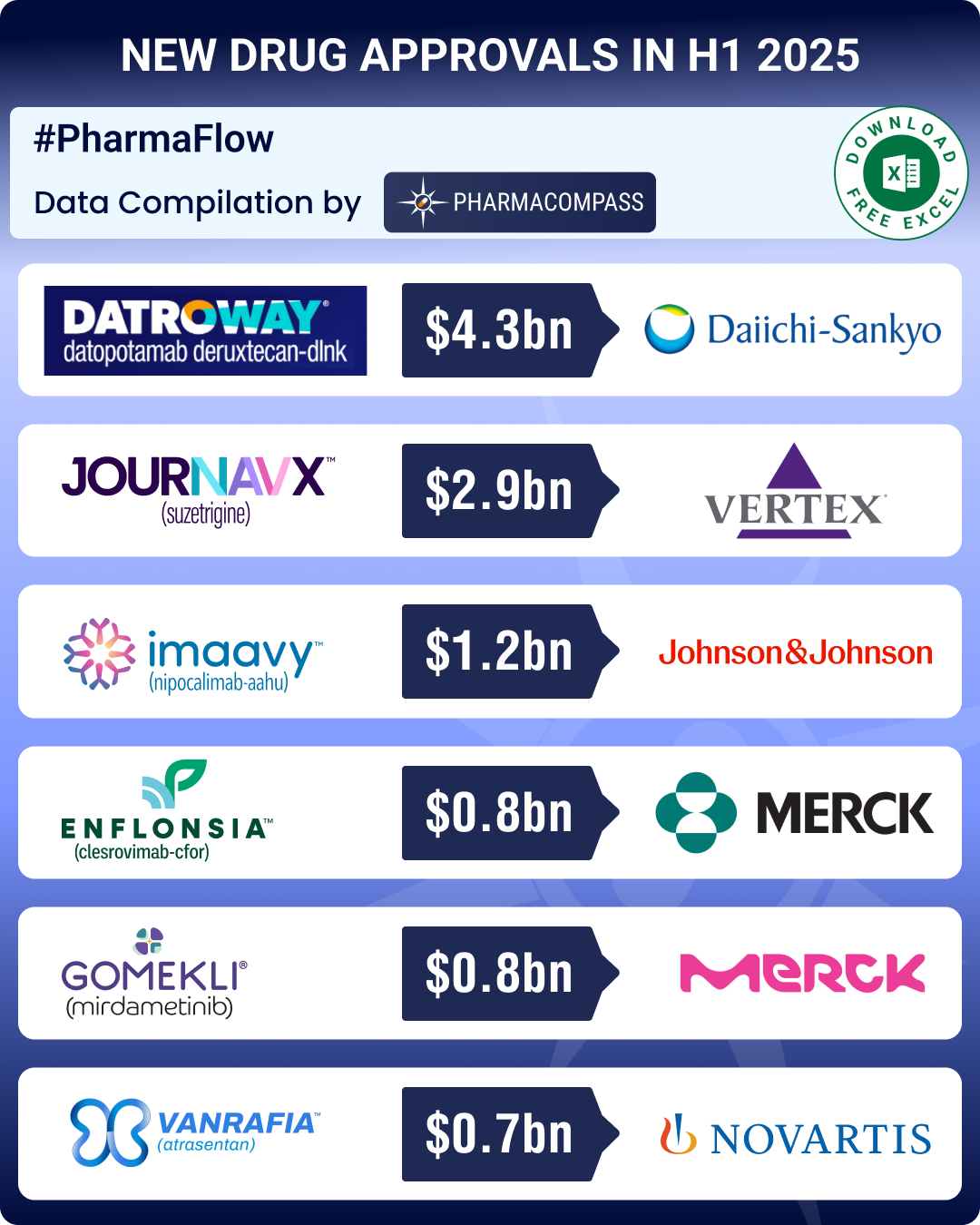
FDA approvals drop 24% in H1 2025; GSK’s UTI med, Vertex’s non-opioid painkiller lead pack of first-in-class meds
It has been a turbulent year for the US
Food and Drug Administration (FDA), marked by reduction
J&J’s Intra‑Cellular buyout, BMS’ oncology gambit, Sanofi’s Blueprint acquisition drive mega deals in H1 2025
The pharmaceutical industry has witnessed a wave of mergers, acquisitions, and strategic partnership
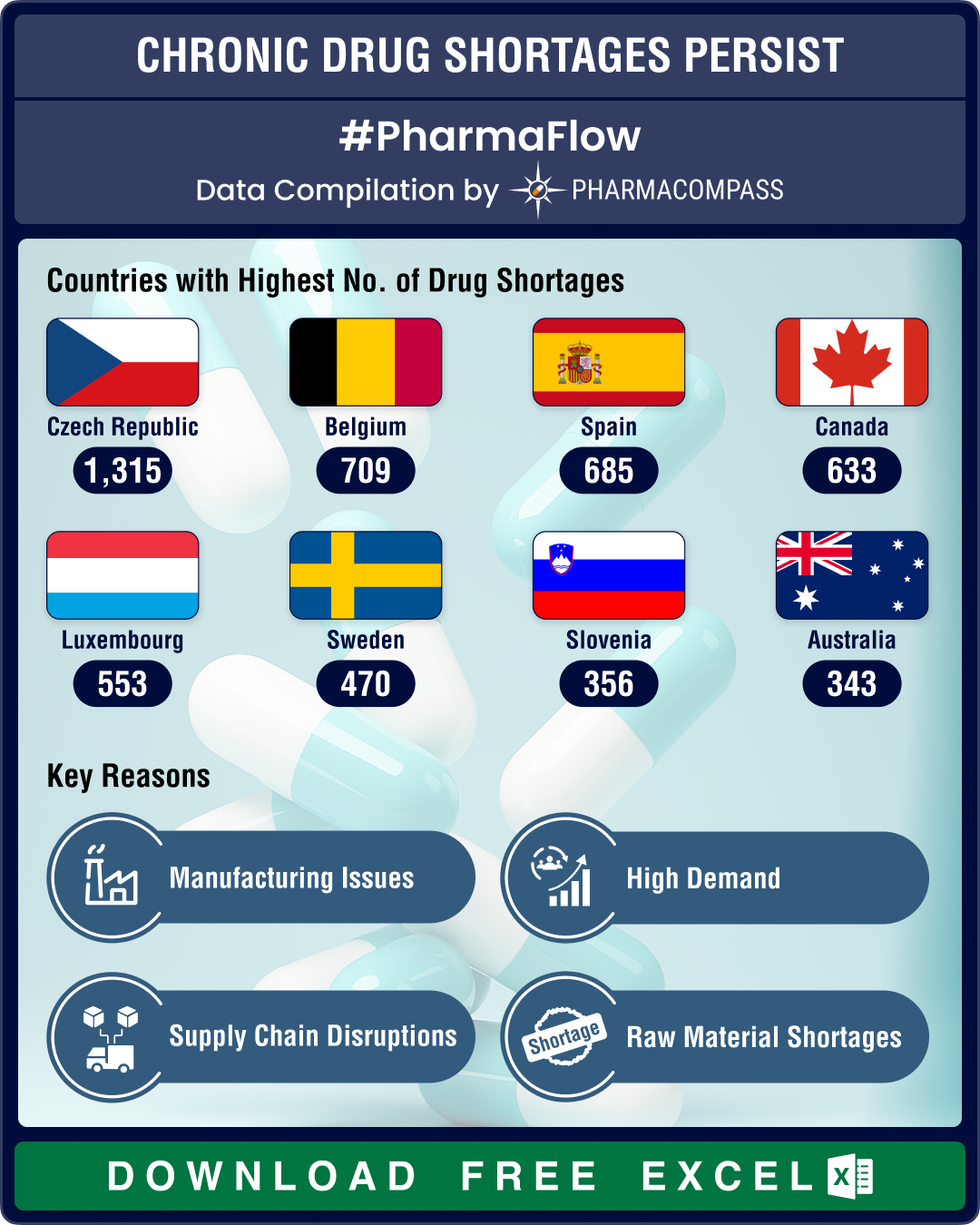
US drug shortages reduce 16% YoY in Q1 2025; CNS drugs, antimicrobials face highest scarcities
The pharmaceutical industry in the United States continued to
grapple with drug shortages during th
Top first-in-class drug candidates of 2025: Ionis’ donidalorsen, Sanofi’s fitusiran, Cytokinetics’ aficamten await FDA approval
First‑in‑class drugs are therapies with entirely new approaches that improve patient out
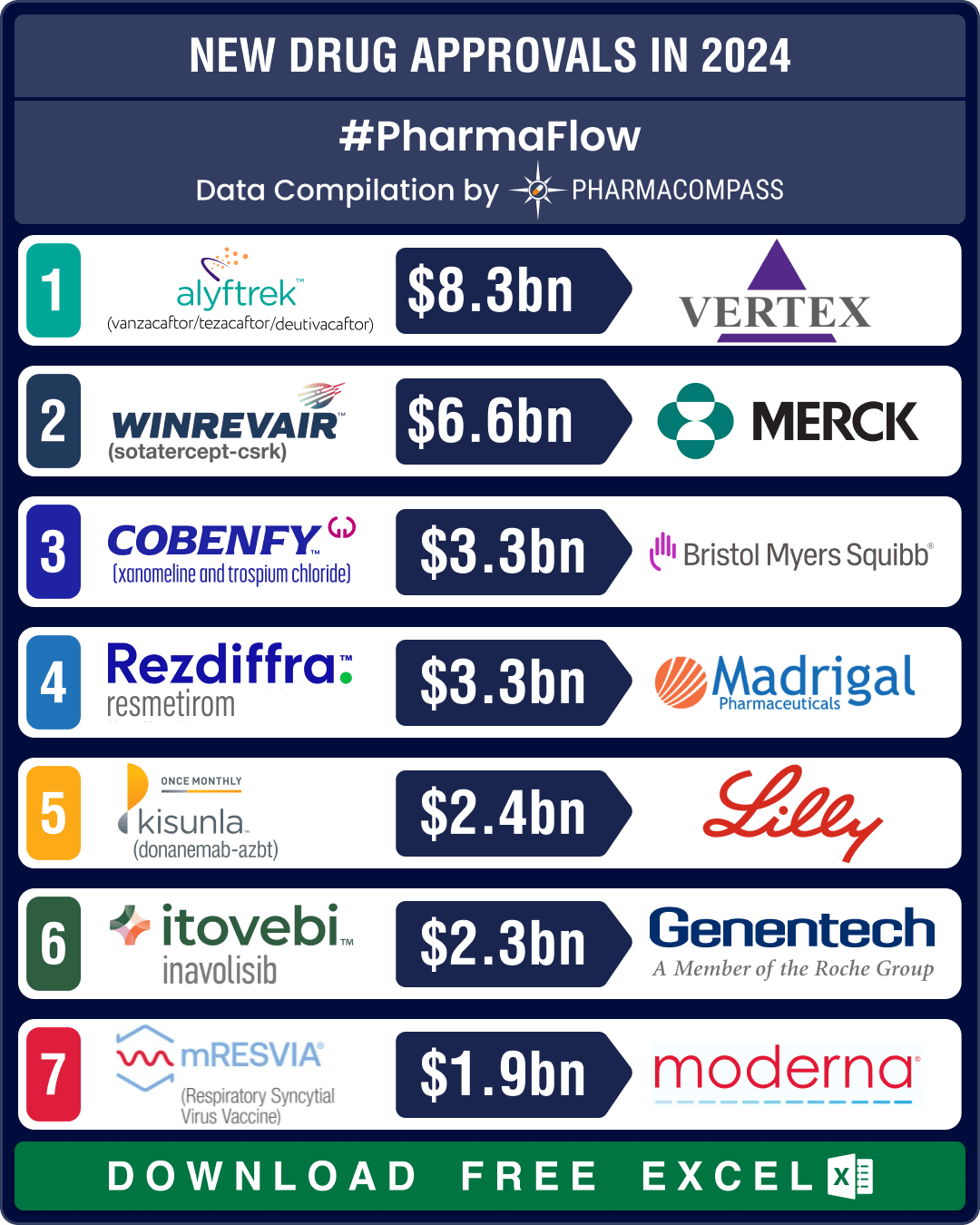
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
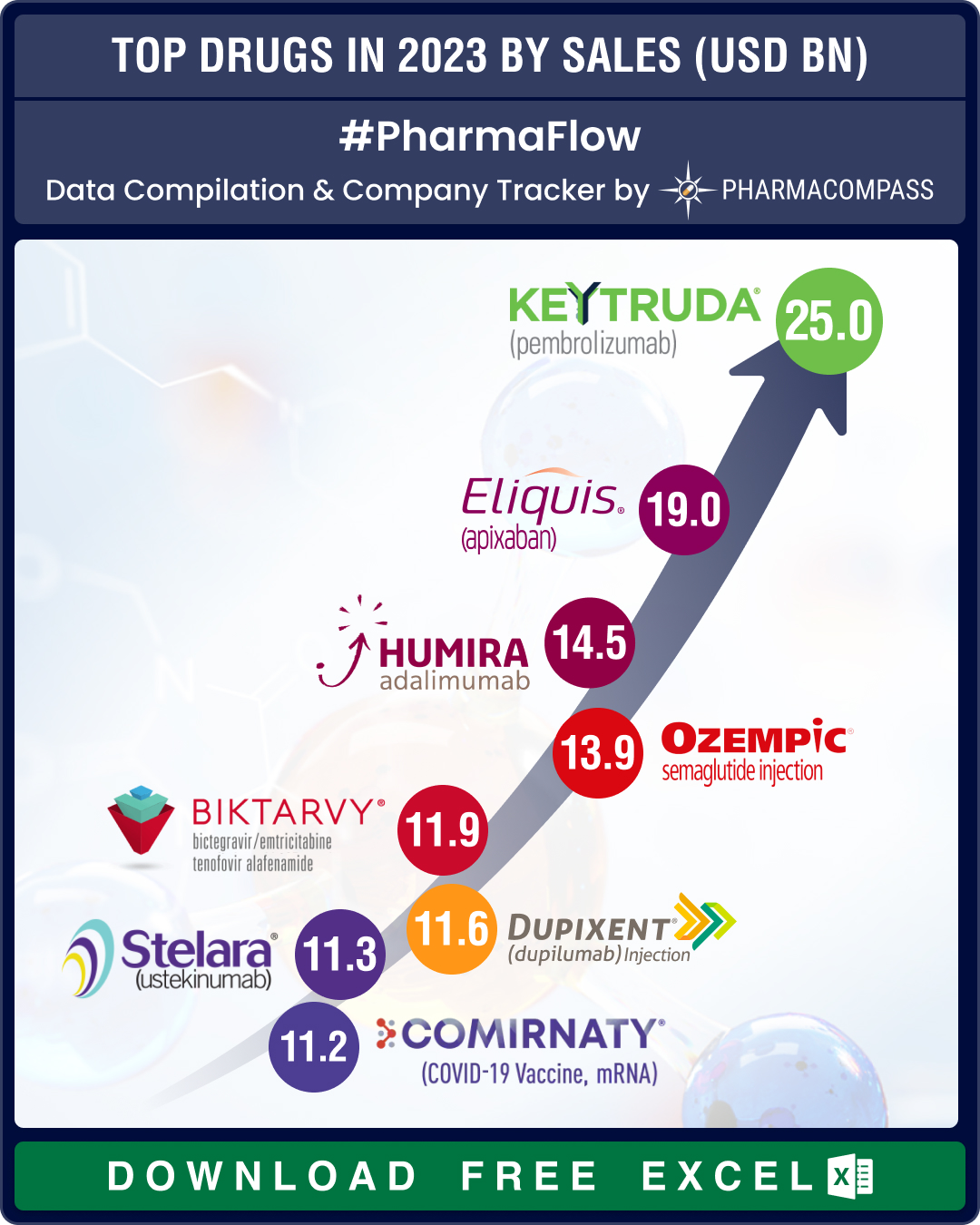
FDA’s landmark approvals of BMS’ schizo med, Madrigal’s MASH drug, US$ 16.5 bn Catalent buyout make it to top 10 news of 2024
The year 2024 was marked by some landmark drug approvals in the areas of schizophrenia, metabolic dy
CDMO Activity Tracker: Bora, PolPharma make acquisitions; Evonik, EUROAPI, Porton announce technological expansions
The contract development and
manufacturing organization (CDMO) space continued to grow at an impres
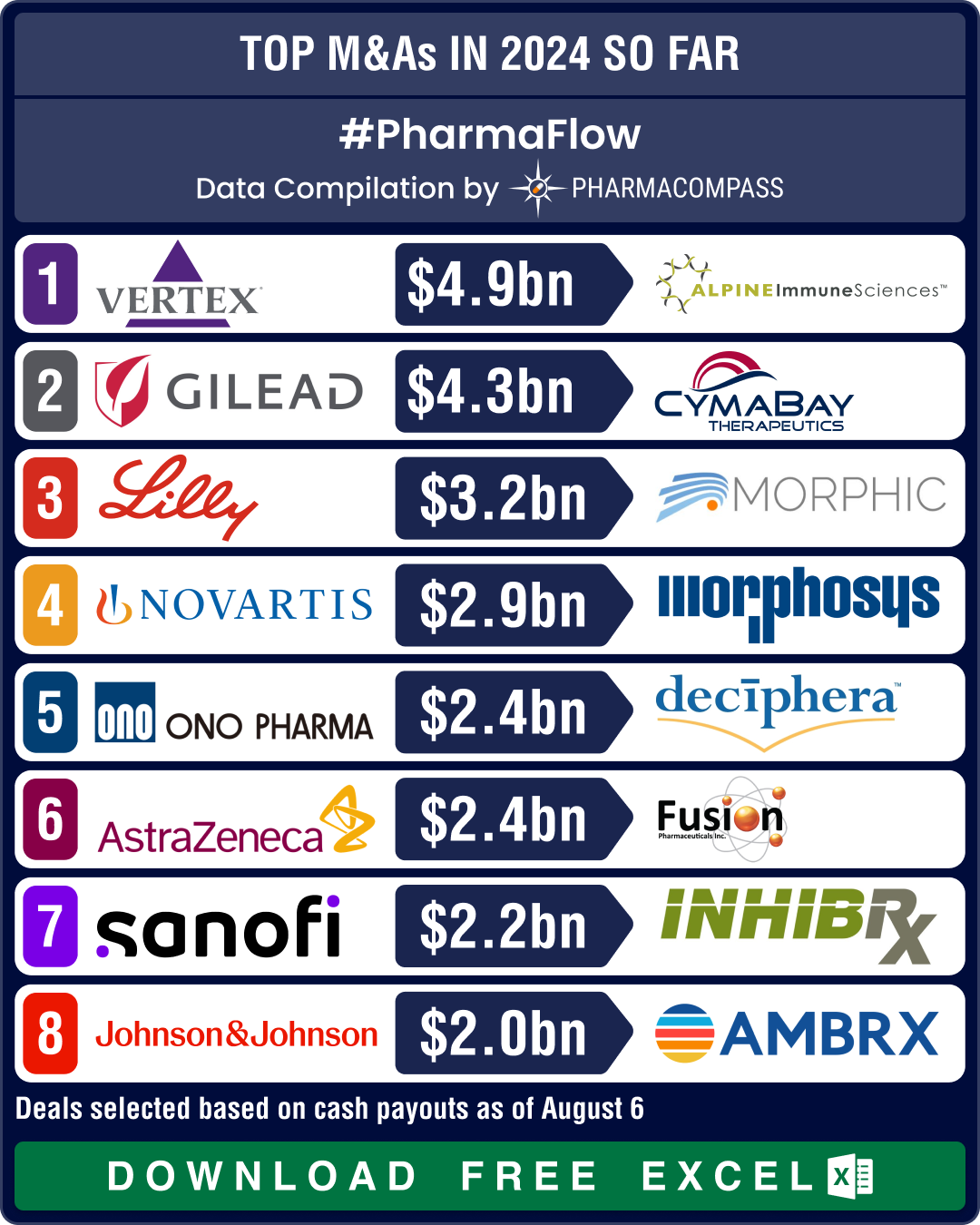
Novartis, GSK, Sanofi, BMS shell out over US$ 10 bn in dealmaking, as mid-size deals take centerstage in 2024
The world of pharmaceuticals and biotechnology continued to evolve
this year with strategic allianc
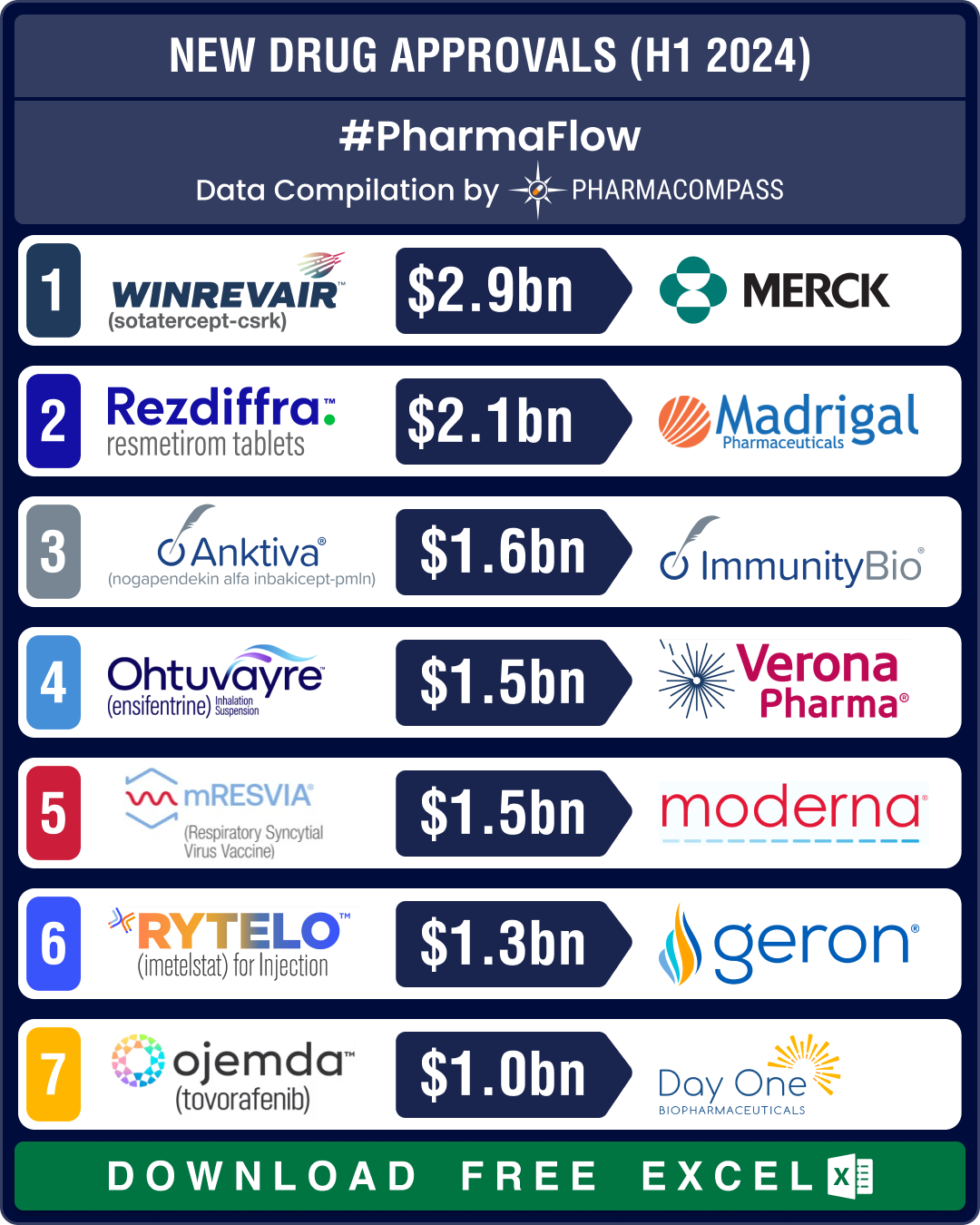
FDA approvals slump 19% in H1 2024; NASH, COPD, PAH get new treatment options
The first half of 2024 saw a significant slowdown in approvals of new drugs and biologics by the US


 Market Place
Market Place Sourcing Support
Sourcing Support