By PharmaCompass
2023-11-09
Impressions: 7436
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect from manufacturers of pharmaceuticals, generic drugs, biosimilars and medical devices in the coming financial year. The fiscal year 2024 fee under the Generic Drug User Fee Act (GDUFA) was published on July 28, 2023.
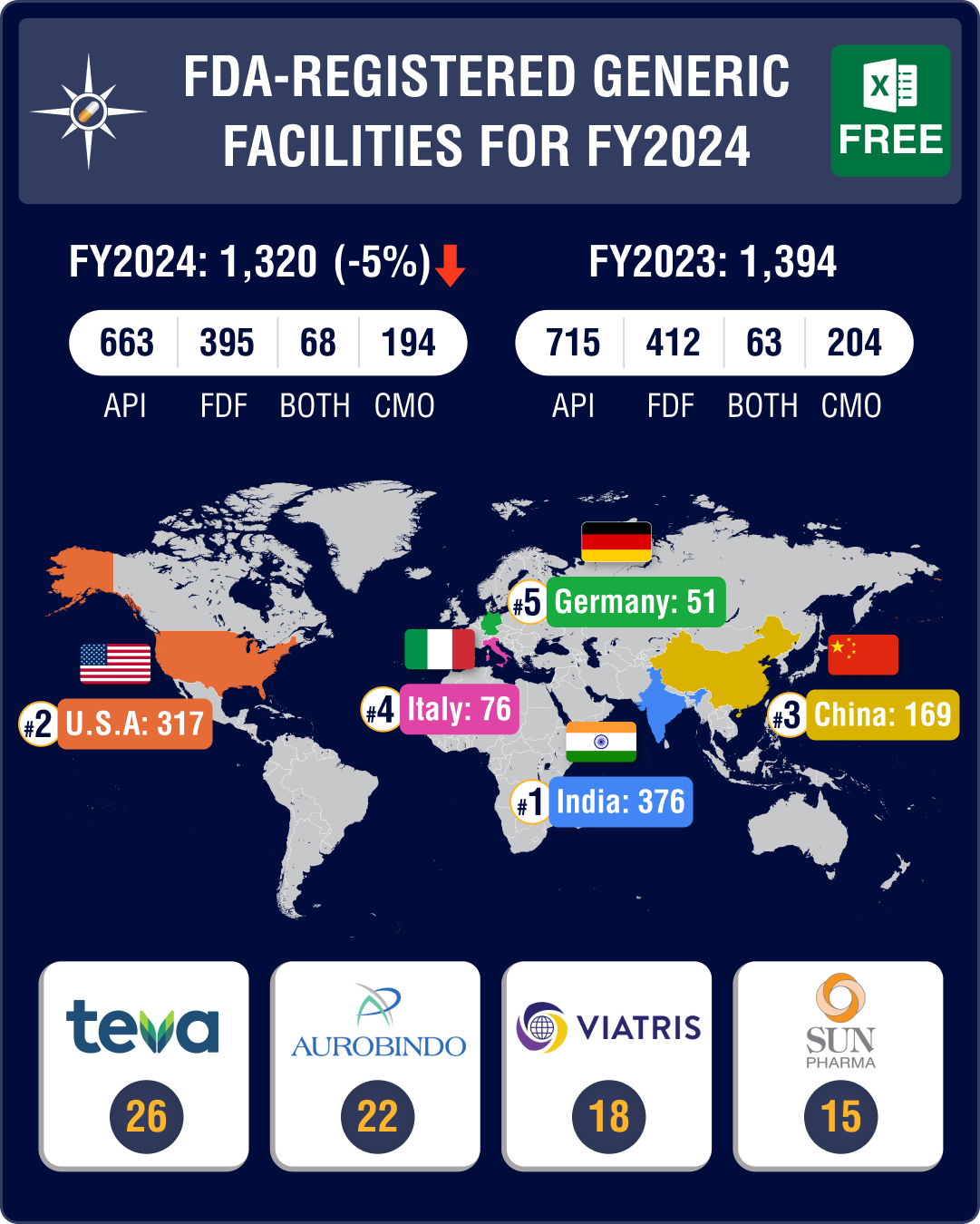
The facility payments list under the GDUFA has revealed that as of October 30, 2023, 1,320 facilities had paid their registration fee for financial year 2024.
Out of this, 663 or 50.2 percent are active pharmaceutical ingredients (API) facilities, 395 or 30 percent are finished dosage forms (FDF) facilities, 68 (5.15 percent) are facilities that produce both APIs and FDFs, and 194 (14.7 percent) are contract manufacturing services (CMO) sites.
Teva Pharmaceuticals led the list of companies by facility registrations, followed by Aurobindo Pharma and Viatris. Teva has 26 facility registrations, including 16 for FDFs, nine for APIs, and one for both APIs and FDFs.
Aurobindo Pharma has 22 facility registrations, including 12 for FDFs, eight for APIs, one for CMO, and one facility that is engaged in both API and FDF activities. Viatris has registered 18 facilities, including 13 for FDFs and four for APIs, and one facility that is engaged in both API and FDF activities.
Generic Drug Facilities Registered with the USFDA in FY2024 (Free Excel Available)
India tops with 376 facility registrations, US comes second at 317
India continues to dominate the list of total facility registrations with the FDA — it registered 376 facilities for FY2024, including 200 API facilities and 134 FDF facilities, 21 facilities engaged in both API and FDF activities, and 21 CMO facilities.
The United States followed India with 317 facilities and China held the third position with 169 facilities.
At 200, India continues to have the largest share of API facilities, which is almost equal to the API sites registered together by both China (112) and the US (76). Amongst European drugmakers, Italy leads with the 54 API manufacturing sites, followed by Spain (30) and Germany (29).
The largest number of facilities for FDFs are in the US (143 sites), followed by India (134) and China (38).
|
Country |
API |
FDF |
Both |
CMO |
Total |
|
India |
200 |
134 |
21 |
21 |
376 |
|
USA |
76 |
143 |
12 |
86 |
317 |
|
China |
112 |
38 |
11 |
8 |
169 |
|
Italy |
54 |
2 |
2 |
18 |
76 |
|
Germany |
29 |
4 |
1 |
17 |
51 |
|
Spain |
30 |
9 |
1 |
4 |
44 |
|
Canada |
7 |
13 |
|
12 |
32 |
|
France |
16 |
1 |
|
7 |
24 |
|
Taiwan |
10 |
5 |
6 |
2 |
23 |
|
Switzerland |
15 |
3 |
|
4 |
22 |
|
Japan |
19 |
|
1 |
|
20 |
Generic Drug Facilities Registered with the USFDA in FY2024 (Free Excel Available)
GDUFA III reauthorization and FDA user fee rates for FY 2024
The GDUFA is a law designed to speed up the access to safe and effective generic drugs for Americans and reduce the costs to the industry. The GDUFA was reauthorized on September 30, 2022 (as GDUFA III), with provisions that came into effect on October 1, 2022, and will last until September 30, 2027.
In July 2023, FDA published user fee rates for FY 2024 for prescription drugs, generic drugs, biosimilars and medical device user fee programs, as well as for outsourcing facilities.
|
Fiscal year |
Facility Registrations |
|
2013 |
1390 |
|
2014 |
1414 |
|
2015 |
1450 |
|
2016 |
1425 |
|
2017 |
1442 |
|
2018 |
1269 |
|
2019 |
1286 |
|
2020 |
1300 |
|
2021 |
1340 |
|
2022 |
1385 |
|
2023 |
1394 |
|
2024 |
1320 |
Last year, FDA had reduced the fee for API facilities and CMOs. However, the FY2024 fee for FDF facilities, both domestic and foreign, has gone up by over 3 percent. Similarly, the fee for large-, medium- and small-sized drug applicants has gone up by over 7 percent.
Generic Drug Facilities Registered with the USFDA in FY2024 (Free Excel Available)
46 new facilities registered in FY24; India leads with 20 new units, 11 set up in China
Out of the total 1,320 facilities registered for FY2024, 46 were new. Out of these, 20 were registered in India, followed by 11 in China and six in the USA.
Out of the 46 new facilities, 22 are FDF facilities, including units of Alembic Pharmaceuticals (in Karkhadi, Gujarat, for injectables and ophthalmic products and in Jarod, Gujarat, for oral solids), Amneal, Aspen (in Gqeberha, South Africa, for oral solids, eye drops and sterile manufacturing), Aurobindo Pharma (in North Carolina, USA, for R&D and manufacturing of inhalation, topical and transdermal products), Novartis (in Ljubljana, Slovenia, for aseptic products, non-aseptic solutions, biosimilars, and nasal spray), Lupin (injectables), Torrent, and Granules India.
There are 13 API units among the list of new registrants (including facilities of players like Global Calcium, Hikal, and Ipca Laboratories), and 11 CMO facilities (including units of Bora Pharmaceuticals and the Hetero Group).
Among the new registered CMOs is Bora Pharmaceuticals’ Mississauga (Canada) facility that produces a range of dosage forms, including oral solid dose (OSD), liquid and semi-solid therapeutics (creams and ointments). Bora had acquired this facility from GSK in 2020.
In FY2024, there were 135 facilities that did not renew their registration. Amongst these were facilities owned by Akorn Pharma, which had shutdown its US operations in early 2023. In fact, 45 of the 135 facilities that did not renew their registrations were from the US.
Generic Drug Facilities Registered with the USFDA in FY2024 (Free Excel Available)
Our view
Over the last few years, the US drug regulator has been contending with regulatory compliance and quality issues of Indian drug manufacturers. America has also been trying to reduce its reliance on China. But the GDUFA facility payments list for FY2024 reveals that not much has changed, and it will business as usual in the coming year.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Generic Drug Facilities Registered with the USFDA in FY2024 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







