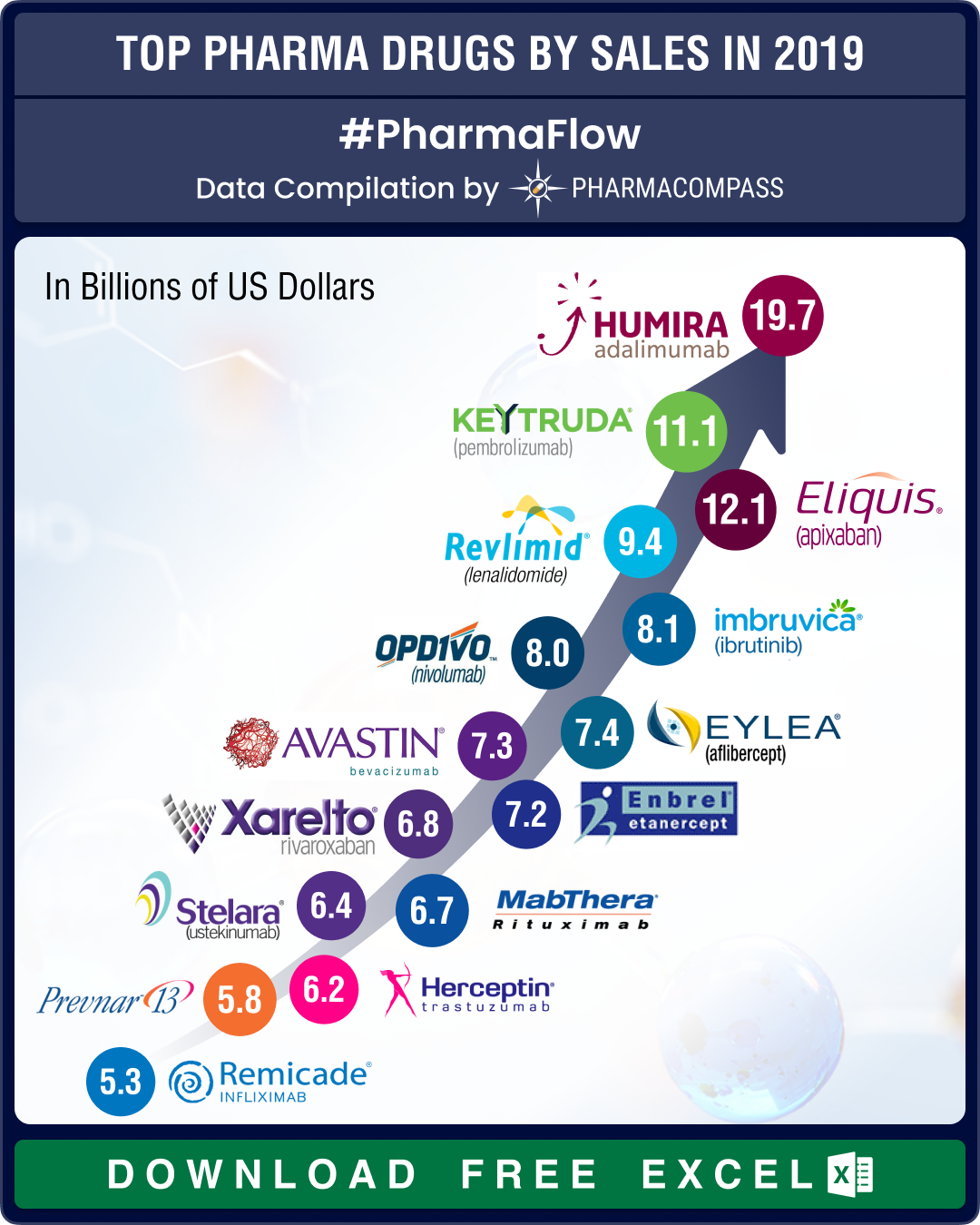
Top drugs and pharmaceutical companies of 2019 by revenues
Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-
Divi’s Labs on FDA’s Import Alert List; but did it refuse an inspection?
Divi's Laboratories — an Indian active pharmaceutical ingredient (API) manufacturer and until
Haunted: Teva’s $1.2 billion ‘pay-for-delay’ penalty; which companies will get hit next?
Teva Pharmaceutical Industries, Ltd., which acquired Cephalon in 2012, will make a total payment of
Pharma Chess: Strategies adopted in the United States to block generics
When a generic drug comes to the U.S.
market, sales of brand drugs crash. The drop is more than 80%


 Market Place
Market Place Sourcing Support
Sourcing Support