Last year, data integrity was a hot topic of discussion in the pharmaceutical industry. According to a recent analysis by GMP (good manufacturing practices) intelligence expert, Barbara Unger, approximately 80 percent of all FDA warning letters in 2015 and 2016 included a data integrity component, and approximately 70 percent of the published European GMP non-compliance reports cited similar shortcomings.
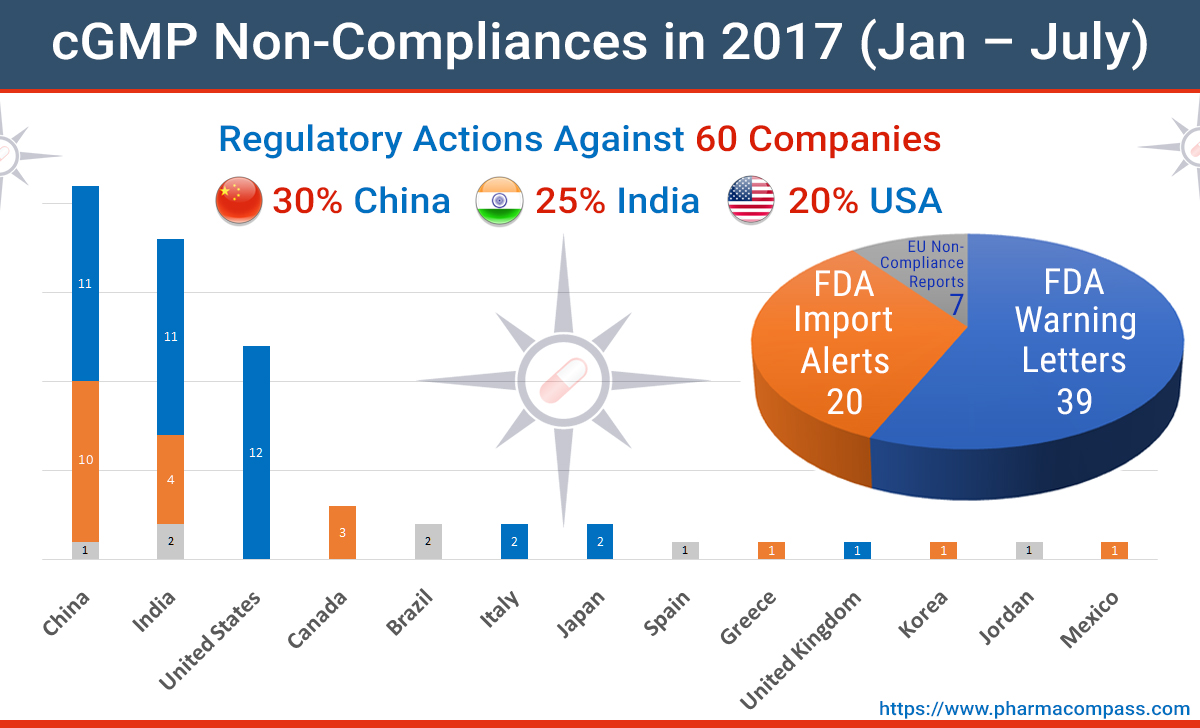
With a little over half the year gone, PharmaCompass analyzed the regulatory action for current GMP (cGMP) non-compliance to evaluate how things are looking so far in 2017.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
As per our analysis, of all the non-compliance actions taken by the US and European regulators, India and China continue to see the highest level of activity, followed by the United States.
While most of the companies in the list are less known pharmaceutical players, inspections uncovered deficiencies at leading companies like Pfizer, Teva, Mylan and B Braun.
Data integrity violations continue to remain high
The US Food and Drug Administration (US FDA) and EU inspections continued to uncover data integrity issues across countries such as India, China, Italy and Japan.
In a warning letter posted earlier this year, for a January 2016 inspection, FDA investigators uncovered data-integrity violations at ACS Dobfar’s Italian drug manufacturing facility — FACTA Farmaceutici SpA. At FACTA, for multiple lots of sterile drug product, the original data showed failing results. However, the final data reportedly showed passing results.
The company was found storing original data in an “unofficial” and uncontrolled electronic spreadsheet on a shared computer network drive. The analyst told investigators that original data was first recorded in the “unofficial” spreadsheet and later transcribed to an “official” form.
Investigators also documented that employees at FACTA used paper shredders to destroy critical laboratory and production records.
During an inspection performed exactly at the same time, FACTA’s EU GMP certification was renewed by the Italian regulators!
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
Discrepancies
in conclusions drawn by regulators
This year, while the United States and the European Union (EU) finally announced that they will be able to utilize each other’s good manufacturing practice (GMP) inspections of pharmaceutical manufacturing facilities, the road ahead looks uncertain as, in addition to incidents like FACTA, there have been multiple instances of discrepancies in the conclusions arrived at by regulators.
This year saw the WHO grant an all clear for a Mylan facility where the FDA had data-integrity concerns and a similar situation arose at an active pharmaceutical ingredient (API) facility in China.
An inspection conducted by the USFDA at Qinhuangdao Zizhu Pharmaceutical from November 28 to December 1, 2016 uncovered significant data integrity concerns and failures in the level of adherence to cGMP for APIs.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
In the warning letter issued to the firm, the laboratory analysts admitted to FDA inspectors that they had been “setting the clock back and repeating analyses for undocumented reasons.”
At Qinhuangdao Zizhu, “initial sample results were overwritten or deleted” and the company “reported only the passing results from repeat analyses”.
On March 8, 2017, Qinhuangdao Zizhu Pharmaceutical was placed on import alert by the USFDA.
Almost a year prior to the USFDA inspection, in October 2015, the company had been inspected by a WHO Prequalification Team (PQT) for levonorgestrel, mifepristone and ethinylestradiol APIs. The inspection concluded with “five major deficiencies, including data integrity issues and several minor deficiencies”.
The WHO went ahead and closed their inspection as ‘compliant’, based on corrective and preventive actions (CAPAs) provided by the manufacturer.
In view of the US FDA actions, and the fact that Qinhuangdao Zizhu Pharmaceutical was the only WHO-PQT prequalified source of levonorgestrel API, as in the case of Mylan, the WHO approach towards the compliance position was focused extensively on product quality.
In Japan, at Sato Yakuhin Kogyo, FDA investigators found analysts performed testing in duplicate without scientific justification, while in China, the French Ministry of Health found manipulation, backdating and falsification of GMP documents such as batch manufacturing records, GC (gas chromatography) and HPLC (high performance liquid chromatographs) chromatograms at Chongqing Succeway Pharmaceutical.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
Heparin makes
headlines again in China; Indian firm fakes strike
This year, concerns over the testing of Heparin in China re-emerged when FDA issued a warning letter to a contract testing laboratory — Shandong Analysis and Test Center. However, the activities at these companies to deceive inspectors paled in comparison to Vikshara Trading in India where the company faked a strike to prevent an FDA inspection.
When the FDA inspection finally occurred, the FDA obtained evidence that the firm actively manufactured numerous products at the time of the supposed strike.
Concerns over
product quality in the United States
In a warning letter issued to ChemRite CoPac in the US, the FDA found that the company was manufacturing oral drug solutions using the same equipment that was being used to manufacture numerous non-pharmaceutical materials including an industrial car care product. The car care product being made was paraffin-based and carried labels such as “harmful or fatal if swallowed” and “keep out of reach of children.”
The ingredients of these non-pharmaceutical products were extremely difficult to remove from the manufacturing equipment, and could contaminate the drug products, the FDA said.
At Raritan Pharmaceuticals, a company that makes teething tablets, the FDA found the drug contained ingredients, such as belladonna, which could pose potential toxic effects for its consumers — infants and children under two years of age.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
Sterile drug
manufacturing continues to be a global challenge
Non-compliant operations uncovered at Sato Pharmaceutical (Japan), Euro Far Allergi (Spain), Tubilux (Italy), Biocon (India), Nova DFL Industrie (Brazil), Pfizer’s US operations (ex-Hospira) show that aseptic sterile drug manufacturing continues to be a global challenge as companies struggle to get into compliance.
In February 2015, Pfizer acquired a site in McPherson, Kansas, through its US$ 17 billion acquisition of Hospira. Pfizer was aware of Hospira's manufacturing record when it struck the deal, as the company was issued FDA warning letters in four of the seven continents — Europe, North America, Asia and Australia.
Approval of two drugs have been held up this year due to compliance concerns at McPherson.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
The USFDA also warned manufacturers of non-sterile, water-based drug products of Burkholderia cepacia complex (BCC or B cepacia) contamination, as there were product recalls due to this and other water-borne opportunistic pathogens found in pharmaceutical water systems.
The regulator’s warning stemmed from a multi-state outbreak of infections. In March this year, Phispers had carried a news item on Badrivishal Chemicals & Pharmaceuticals, a manufacturer of docusate sodium. It had been placed on FDA’s import alert list in December last year.
Concerns over water systems were also mentioned in the warning letters issued to Humco Holding Group in the United States and Resonance Laboratories in India.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
Omitting names of
original suppliers; shipping drugs from banned API makers
The USFDA investigators found companies in India (Sal Pharma) and China (Suzhou Pharmaceutical Technology and Lumis Global Pharmaceuticals) omitting the name and address of the original API manufacturers on certificate of analysis and declared themselves to be the manufacturers. In the case of Suzhou, one of its suppliers was placed on FDA’s import alert list. However, the company shipped API manufactured from the banned supplier by providing misleading declarations.
Last year, PharmaCompass had reported on Teva’s newly built sterile manufacturing facility in Godollo, Hungary, and the issues highlighted by the FDA in its warning letter and the product recalls from this unit. As part of its global restructuring, while Teva is now winding up its sterile injectables plant in Godollo, it remains to be seen what decision will be taken on its API manufacturing facility in Hangzhou (China), where the FDA had highlighted concerns over process capability and the resulting impact on product quality.
Wockhardt’s global compliance problems also continued with its manufacturing facility in the United States — Morton Grove Pharmaceuticals — receiving a warning letter.
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
Our view
Earlier this year, a major Indian API manufacturer — Divi’s Laboratories — was placed on FDA’s import alert list. It was issued a warning letter due to a variety of problems which were uncovered at the site. The concerns raised at Divi’s along with other companies indicate a shifting focus on part of the FDA investigators from audit trails as there is greater depth in the nature of the observations.
To assist the industry, the FDA has started posting frequently requested Form 483s on a routine basis which provides insight for the industry to track the new areas of regulatory focus.
You can find the Form 483s on PharmaCompass at https://www.pharmacompass.com/radio-compass-news/Quality-Alerts
Click here to access the compilation of all 2017 non-compliances (Excel version available) for FREE!
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : cGMP Non-Compliances in 2017 (Jan - July) by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”






