Amgen ends Humira’s 20-year reign in US with Amjevita launch
This week, AbbVie’s Humira (adalimumab) saw its 20-year exclusive
run come to an end in the U
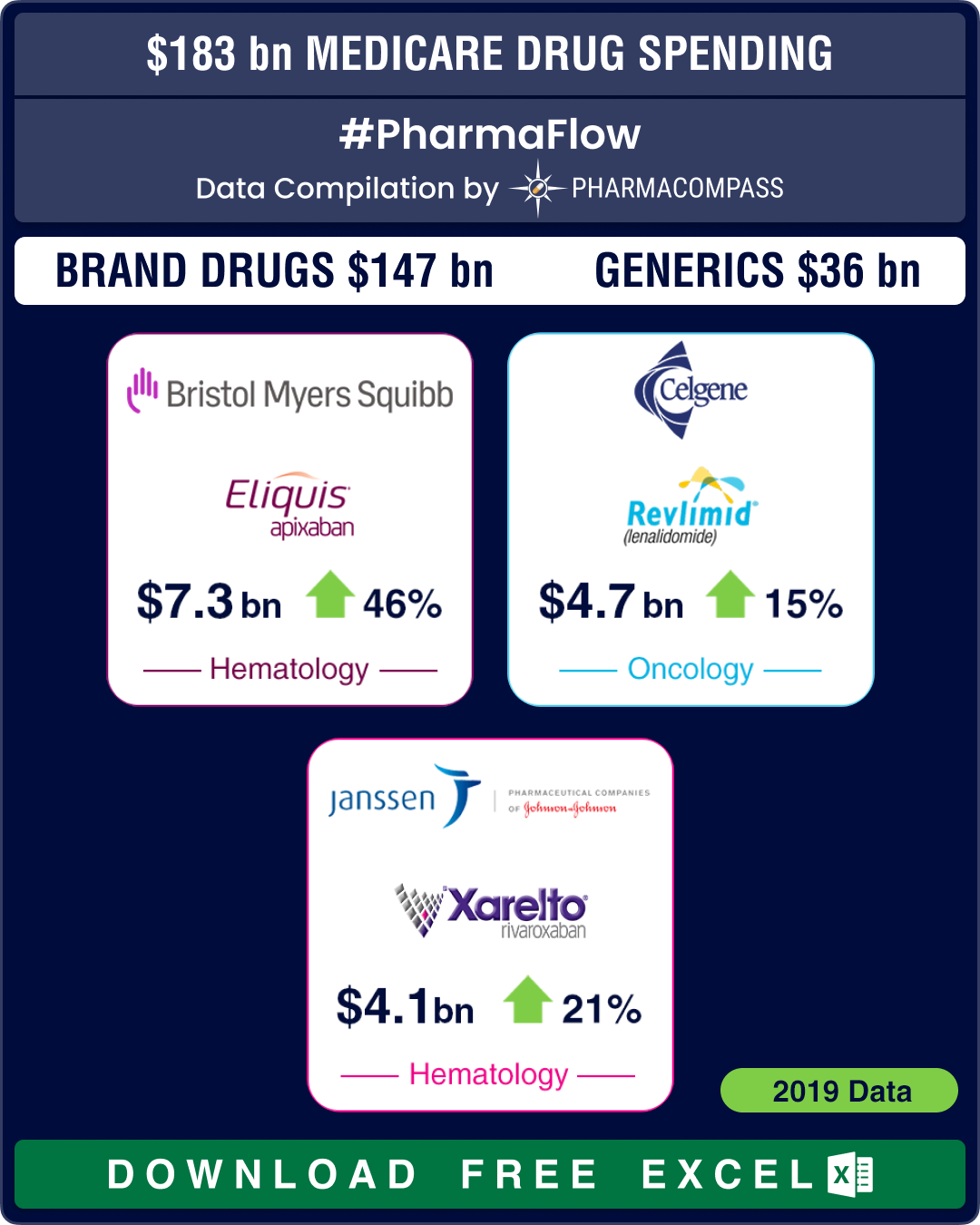
America’s drug price hike conundrum in backdrop of 2019 Medicare Part D data
Nearly every
year, drugmakers ring in the new year with drug price increases in the US. This
year
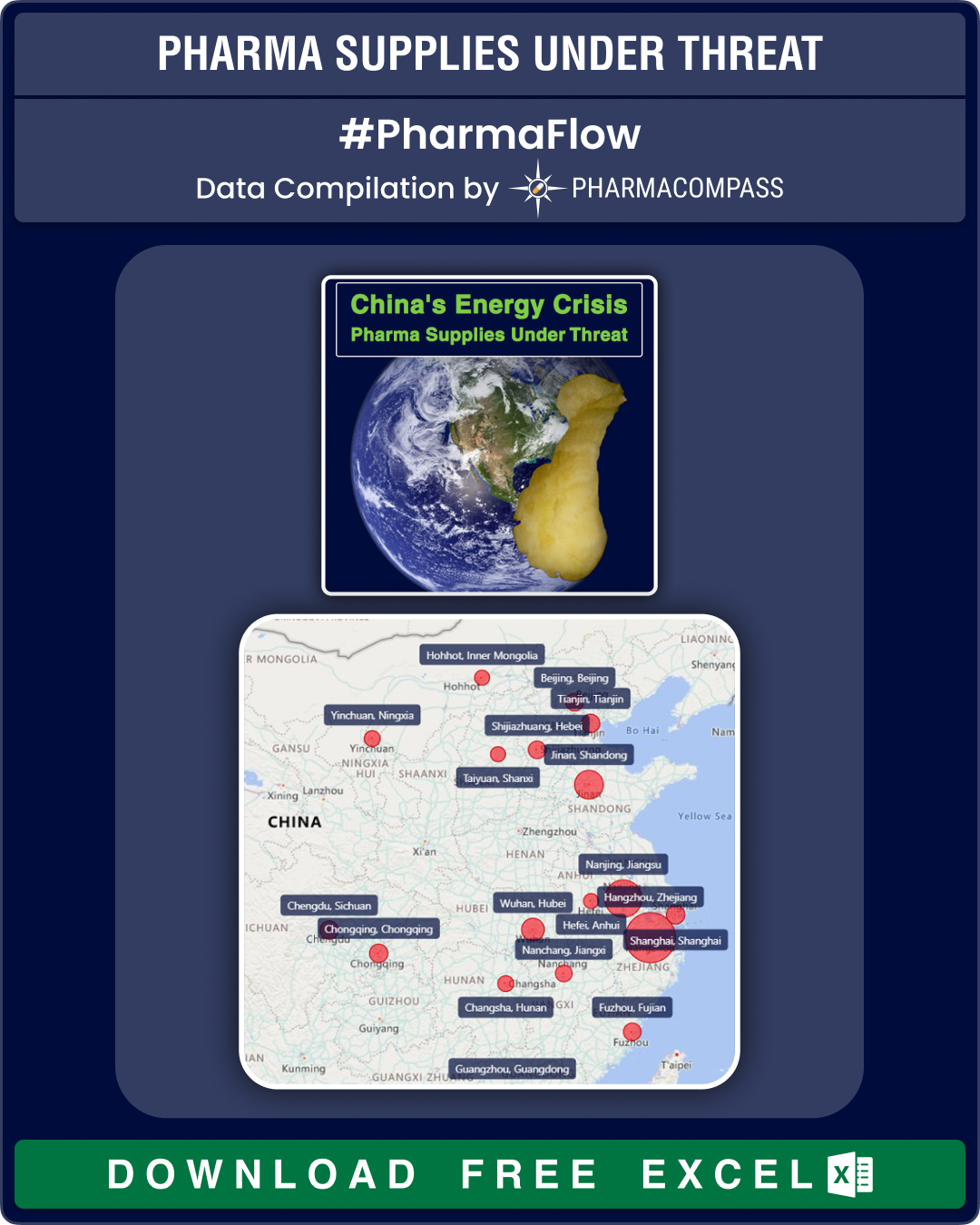
China’s energy crunch threatens pharma supply chains
This
week, PharmaCompass looks at the energy crunch in China and its likely fallout
on the global
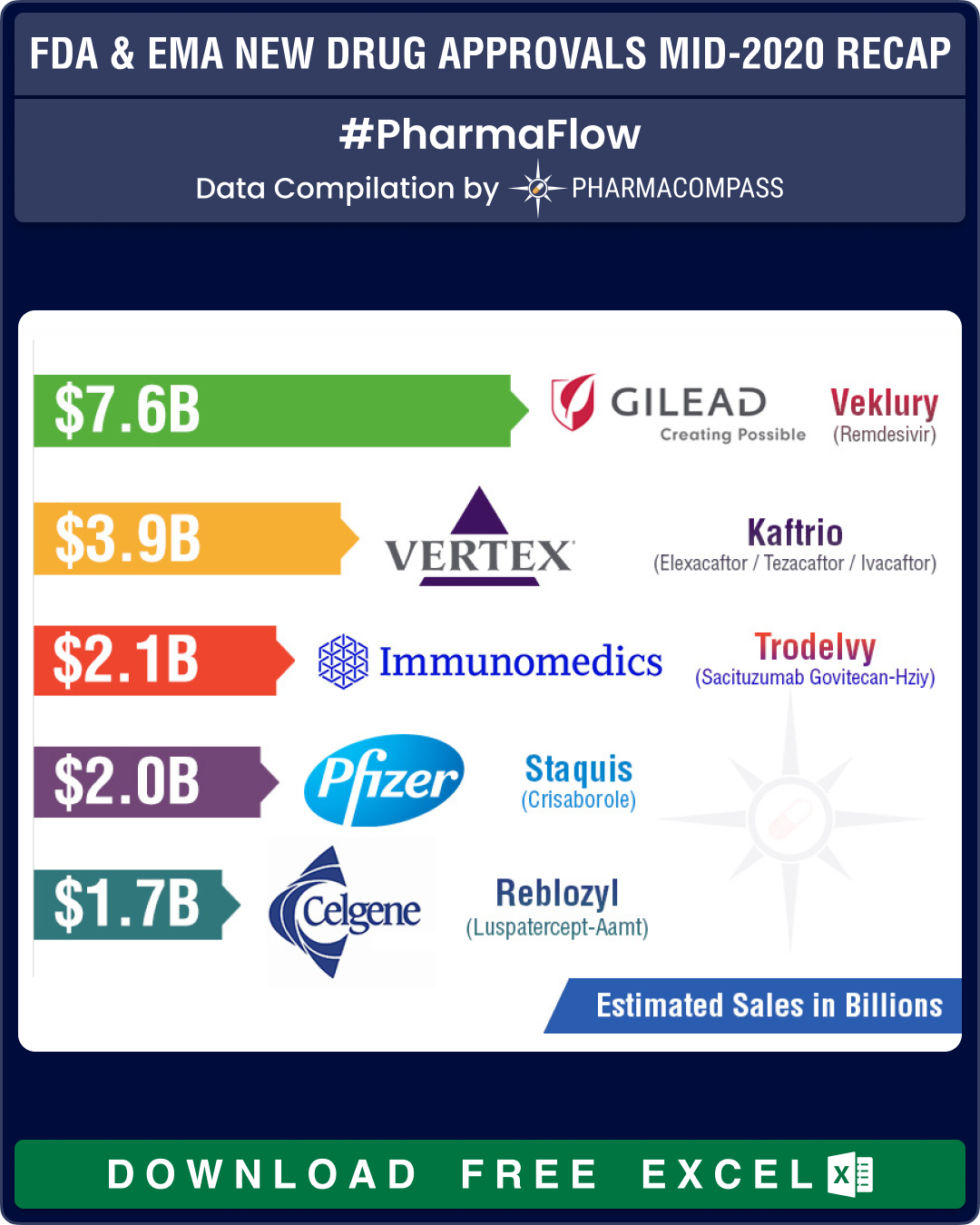
New Drug Approvals by FDA & EMA (Mid-2020 Recap)
In case you thought Covid-19 had slowed down US Food and Drug Administration’s New Drug Approv
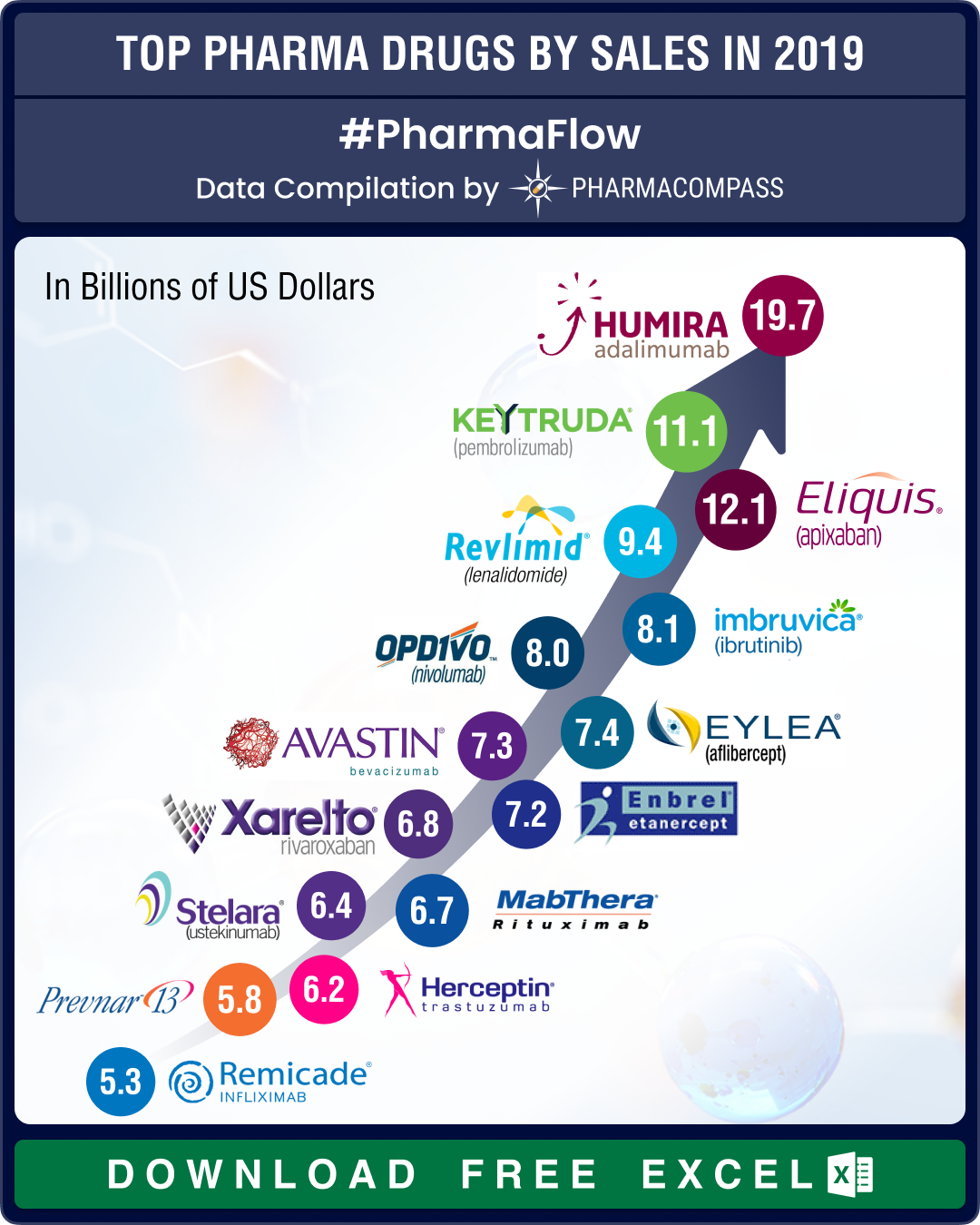
Top drugs and pharmaceutical companies of 2019 by revenues
Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-
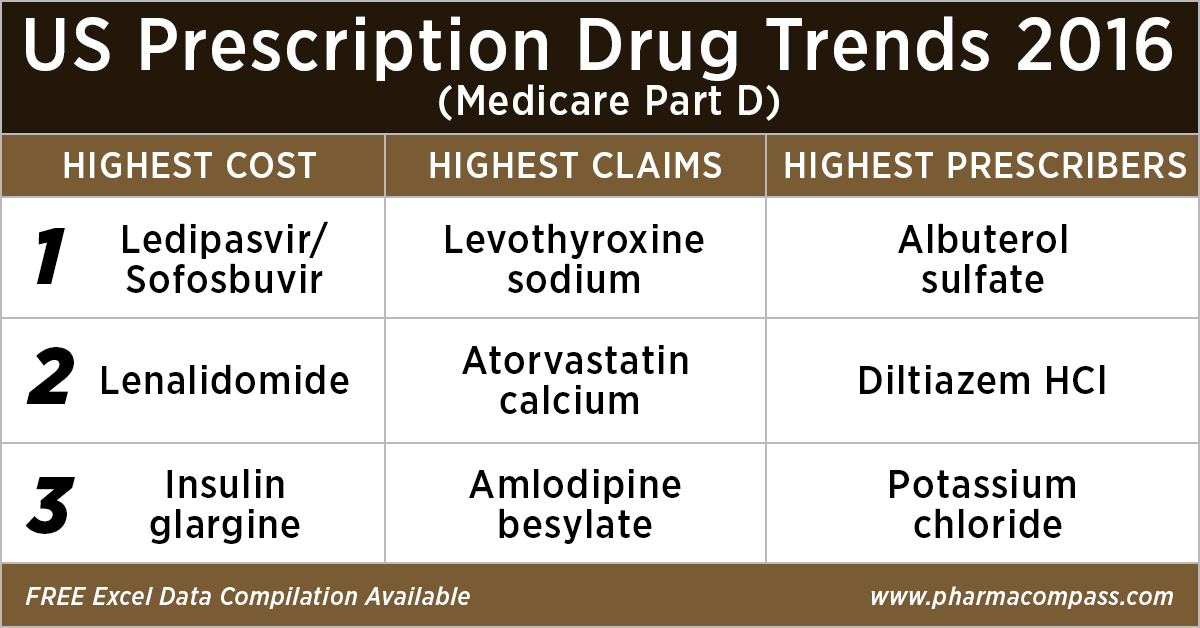
Analyzing over US$ 90 billion of Medicare Prescription Drug (Part D) Spending in 2016
This week, PharmaCompass
reviews the recently released data on prescription drugs paid for under th
Seven of your facilities are out of compliance, FDA tells Wockhardt; Mylan’s EpiPen sales crash
In Phispers this week, there is news on how alternatives to Mylan’s EpiPen have eaten into it
Drug costs and prescription trends in the United States: Analyzing Medicare’s $121 billion spend
In less than three weeks, Donald Trump will assume office as the
President of the United States. He
Compliance Wrap: Heparin quality concerns re-emerge in China, more trouble at Sun Pharma & Wockhardt
PharmaCompass looks at recent compliance concerns highlighted by the US FDA at drug manufacturers li



 Market Place
Market Place Sourcing Support
Sourcing Support