By PharmaCompass
2021-08-11
Impressions: 9267
As regulatory agencies got used to operating amidst the Covid-19 pandemic, New Drug Approvals (NDAs) by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) during the first half of this year witnessed a significant uptick as compared to the same period last year.
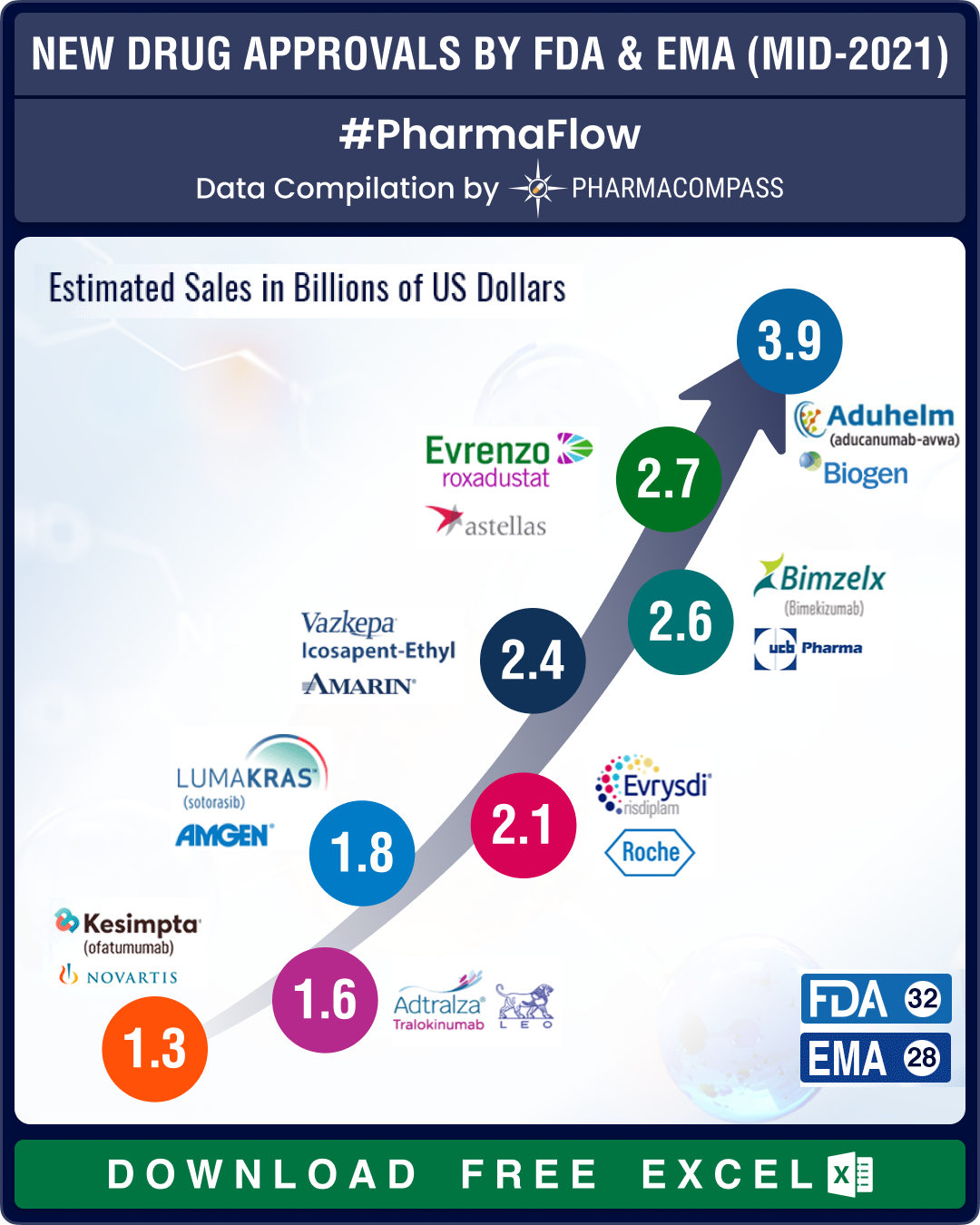
Until June 30 this year, the FDA had approved 32 while the EMA had approved 22 new drugs. Furthermore, to ensure additional treatments were available for Covid patients, the FDA also gave emergency use authorizations (EUAs) to five drugs. Almost one-third of the NDAs were for oncology (18), followed by genetic diseases (seven), and cardiovascular and immunology (at five each).
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
FDA’s controversial approval of Biogen’s Alzheimer’s drug – aducanumab
The year was also mired in controversy as the FDA granted accelerated approval to Biogen’s controversial Alzheimer’s drug — aducanumab — the first treatment to attack a likely cause of the progressive neurological disorder.
While aducanumab — which will go by the brand name Aduhelm — tops our list of new drugs with the highest sales potential (US$ 3.908 billion), its approval proved to be a controversial one since experts are of the view that there is not enough evidence that aducanumab can address cognitive symptoms.
The approval was followed by news that the FDA’s acting commissioner, Janet Woodcock, was taking the unusual step of asking for a federal investigation of the doctors within the FDA who met with Biogen executives before the medicine’s approval.
According to a Wall Street Journal story, the FDA reviewers met people from Biogen and collaborated with the company to prepare a joint review document presented to an FDA panel of external advisers at a public meeting in 2020.
Days after Woodcock’s request, two high-ranking Congressional Democrats were learnt to be seeking answers to several concerns over Aduhelm, which comes at a lofty annual list price of US$ 56,000. In their letter, the lawmakers told Biogen that they are concerned about reports of the company’s “atypical” approval process and a secret campaign, dubbed “Project Onyx.”
In a JAMA Network publication, the FDA highlighted that two randomized, double-blind placebo-controlled, phase 3 studies on aducanumab (301 and 302), which included 1,647 and 1,638 participants, had provided conflicting results.
“Although evidence from the aducanumab program was suggestive of clinical benefit—strongly so in study 302 with support from study 103—we concluded, as did the FDA’s Peripheral and Central Nervous System Advisory Committee, that the clinical trial data were not adequate on their own to convincingly demonstrate a clinical benefit in reducing clinical decline in patients with Alzheimer disease,” the medical journal read.
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
Amgen’s Lumakras — first approved treatment for non-small cell lung cancer
The second drug on the list of FDA approvals with the highest sales potential is Amgen’s Lumakras (US$ 1,756 million).
The FDA approved Lumakras (sotorasib) as the first treatment for adult patients with non-small cell lung cancer (NSCLC) whose tumors have a specific type of genetic mutation called KRAS G12C, and who have received at least one prior systemic therapy.
The agency approved Lumakras three months ahead of schedule. This is the first approved targeted therapy for tumors with any KRAS mutation, which accounts for approximately 25 percent of mutations in NSCLC.
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
BMS, bluebird bio finally bag FDA nod for ide-cel, a multiple myeloma therapy
In March this year, Bristol-Myers Squibb (BMS) and bluebird bio’s CAR T-cell therapy for multiple myeloma — ide-cel — finally bagged FDA approval in the US.
Celgene had entered into an agreement with bluebird bio in 2018 to co-develop and co-promote bb2121 (ide-cel), an investigational anti-B-cell maturation antigen (BCMA) chimeric antigen receptor (CAR) T cell therapy, for the potential treatment of patients with relapsed/refractory multiple myeloma in the United States. BMS merged with Celgene at the beginning of 2019, and as a result of this US$ 74 billion mega merger, bluebird bio began developing ide-cel along with BMS.
In March 2020, BMS and bluebird bio submitted the Biologics License Application (BLA) for ide-cel with the FDA. However, in May 2020, the companies received a refusal to file letter from the FDA regarding the BLA for ide-cel. The two companies then resubmitted the BLA in July 2020.
Post the acquisition by BMS, Celgene shareholders held US$ 9 per share Contingent Value Rights (CVR), which hinged on US regulatory approval of three drugs: Zeposia, ide-cel and liso-cel. Celgene’s multiple sclerosis drug Zeposia (ozanimod) was approved in March 2020. However, the FDA did not approve liso-cel (another CAR-T therapy) by the December 31, 2020 deadline and as a result, the CVR agreement got terminated. The liso-cel therapy bagged FDA approval in February this year. And in March, the FDA approved ide-cel.
Acting as a trust for Celgene’s former shareholders, Kansas City-based UMB Bank NA filed a lawsuit in June this year, wherein UMB alleged that BMS breached the CVR agreement by failing to apply “diligent efforts” to secure the approval, and has also blamed BMS for “blatant misconduct”. UMB is looking for US$ 6.4 billion in restitution.
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
Vazkepa, Evrysdi and Adtralza lead EMA approvals
The leading drugs by potential sales approved by the EMA in the first half of the year were Vazkepa, Evrysdi and Adtralza.
In December 2019, Amarin had received expanded-use FDA approval for Vascepa (sold as Vazkepa in Europe), a drug that reduces cardiovascular risks. Vascepa is a type of omega-3 fatty acid, a fat found in fish oil. However, in March 2020, Amarin lost a major patent battle in the Nevada district court against Hikma and Dr Reddy’s. This opened the door for generic competition to Vascepa — Amarin’s potential blockbuster. Amarin then appealed against the decision.
In May 2020, Hikma received FDA approval for its generic equivalent to Vascepa and a month later Amarin struck a deal with Apotex, one of the generic companies that was not party to the patent case, to put off its Vascepa generic until 2029 if Amarin won its appeal. This was followed by Dr Reddy’s generic Vascepa also bagging the FDA nod.
By September last year, matters had worsened for Amarin when the US Court of Appeals for the Federal Circuit upheld Nevada district court’s ruling that patents protecting Amarin’s Vascepa from generic challengers were invalid.
This ruling dashed Amarin’s hopes of Vascepa becoming a blockbuster cardiovascular drug. However, with the European launch it remains to be seen how well the drug performs with generics in active pursuit of the market.
Evrysdi (risdiplam), approved by the FDA in 2020, is the first oral medicine approved for the rare genetic disease, spinal muscular atrophy, which until four years ago had no available treatments.
The approval of Evrysdi last year presented patients and their families with a unique choice between a one-time gene therapy, an RNA-based drug infused three times a year at the doctor’s office, and a daily medicine taken at home.
While Vazkepa and Evrysdi were approved by the FDA prior to 2021, Leo Pharma’s dermatology treatment Adtralza, which received EMA approval in 2021, faced a setback in the US as the FDA hit Leo Pharma with a Complete Response Letter (CRL) over its eczema drug in April 2021.
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
Our view
The controversial approval of Biogen’s Alzheimer’s drug Aduhelm has prompted the Department of Health and Human Services (HHS) Office of the Inspector General to announce that they will review the FDA’s accelerated approval pathway — a route that allows drugs for serious diseases without existing treatments to be approved if they hit certain interim benchmarks (called surrogate endpoints).
These drugs are thought to provide clinical benefit, but that benefit isn’t actually demonstrated before the drug is approved. Once the drug is approved, a phase four study is required to demonstrate clinical efficacy.
“The FDA’s approval of Aduhelm raised concerns due to alleged scientific disputes within the FDA, the advisory committee’s vote against approval, allegations of an inappropriately close relationship between the FDA and the industry, and the FDA’s use of the accelerated approval pathway,” the announcement read.
It remains to be seen how the investigation impacts the FDA’s new drug approval process in the future.
View New Drug Approvals by June 2021 with Estimated Sales (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA-&-EMA’s-New-Drug-Approvals-Mid-2021-Recap by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








