By PharmaCompass
2022-01-20
Impressions: 7091
The year 2021 was undoubtedly an extraordinary one for both regulatory agencies and the global pharmaceutical industry. Throughout 2021, the Covid-19 pandemic continued to present significant challenges for regulatory agencies across the world. Despite these odds, the US Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 50 new drugs,
either as new molecular entities (NMEs) under new drug applications (NDAs), or as new
therapeutic biologics under biologics license applications (BLAs). This number was down only
around 6 percent from 2020.
Moreover, FDA’s Center for Biologics Evaluation and Research (CBER) approved 10 drugs, including the landmark mRNA vaccine from Pfizer-BioNTech (approved in August) and CAR-T cell products, such as Bristol-Myers Squibb’s Abecma (ide-cel) and Breyanzi (liso-cel).
The European Medicines Agency (EMA), on the other hand, gave marketing authorizations to 49 and conditional authorizations to four drugs.
The FDA’s list of approvals contains several breakthrough therapies, including the first KRAS inhibitor for cancer (Amgen’s Lumakras) and the first anti-amyloid antibody for Alzheimer’s disease (Biogen’s Aduhelm).
View New Drug Approvals in 2021 with Estimated Sales (Free Excel Available)
Year of expedited programs for drug approvals
Interestingly, 2021 was a year of expedited programs. In fact, 37 of the 50 novel drugs approved in 2021 (74 percent) used one or more expedited programs, such as a fast track designation, breakthrough therapy designation, priority review, and/or an accelerated approval.
These pathways allowed shorter review times to speed up the availability of new therapies to patients with serious conditions, given the lack of satisfactory alternative therapies.
The CDER identified 27 of the 50 novel drugs
approved in 2021 (54 percent) as first-in-class, or drugs that have mechanisms
of action different from those of existing therapies. These include drugs like
Aduhelm, Cosela injection (a drug that reduces the
frequency of chemotherapy-induced bone marrow suppression in certain adults
being treated for extensive-stage small cell lung cancer) and Livtencity capsules (used to treat adults
with post-transplant cytomegalovirus infection).
Moreover, 26 of CDER’s 50 novel drug approvals (52 percent) were approved to treat rare or “orphan” diseases (or diseases that affect fewer than 200,000 people in the US). Patients with rare diseases often have few or no drugs available to treat their conditions. Some examples of these drugs are Amondys 45 (treatment for rare Duchenne muscular dystrophy mutation), Besremi (to treat adults with a blood disease known as polycythemia vera), Bylvay (to treat pruritus in progressive familial intrahepatic cholestasis, a disorder that causes progressive liver disease), Empaveli (treatment for adults with a serious, rare blood disease), Lumakras, Nexviazyme (treatment for late-onset Pompe disease) and Vyvgart (for the treatment of generalized myasthenia gravis).
View New Drug Approvals in 2021 with Estimated Sales (Free Excel Available)
Cancer drugs dominate new approvals
Oncology
drugs continued to dominate FDA approvals, accounting for 15 (30 percent) of
the new approvals. The five-year average for cancer approvals is 28 percent.
Four new drugs were approved for non-small cell lung cancer, including one non-small cell lung cancer type (KRAS G12C) previously thought to be resistant to treatment. The four drugs include J&J’s Rybrevant (amivantamab) and EMD Serono’s Tepmetko (tepotinib).
The approval of Amgen’s KRAS-G12C inhibitor — Lumakras (sotorasib) — was an important milestone for oncology. This is the first treatment for adult patients with non-small cell lung cancer (NSCLC) whose tumors have a specific type of genetic mutation, known as KRAS G12C, and who have received at least one prior systemic therapy. The therapy was approved three months ahead of schedule. Amgen is testing sotorasib in combination with other agents, in various cancers.
The FDA has approved five CAR-T therapies, all of which hunt and destroy CD19-expressing cells for blood cancers, including Bristol Myers Squibb’s Abecma (idecabtagene vicleucel), a cell-based gene therapy to treat adult patients with multiple myeloma, and Breyanzi (lisocabtagene maraleucel), which is a cell-based gene
therapy to treat adult patients with certain types of large B-cell lymphoma.
Neurology drugs secured the second most approvals for the third consecutive year, with five (10 percent) new entrants. Using the accelerated approval pathway, CDER approved Aduhelm — a new drug to treat Alzheimer’s disease. This drug is the first therapy that targets the fundamental disease pathophysiology. CDER also approved Abbvie’s Qulipta (atogepant), a drug that helps reduce the frequency of migraine attacks in patients with episodic migraine.
Infectious diseases and cardiovascular diseases saw four approvals each and tied for the third position, followed by psychiatry, immunology and genetic diseases at the fourth position with three drug approvals each.
View New Drug Approvals in 2021 with Estimated Sales (Free Excel Available)
Stars of 2021 — Covid-19 vaccines
Several drugmakers sought to get their Covid-19 therapies and vaccines authorized by the FDA in order to bring an end to the raging pandemic — which has so far claimed the lives of over 55.65 million people worldwide.
Besides vaccines and anti-viral drugs like Merck-Ridgeback’s Molnupiravir and Pfizer’s Paxlovid, the year saw several antibody drugs bag FDA’s emergency use authorization (EUA) — such as Eli Lilly’s Covid-19 antibody combo of bamlanivimab and etesevimab and GSK’s antibody treatment, sotrovimab. In all, the FDA granted EUAs to eight Covid therapies and vaccines.
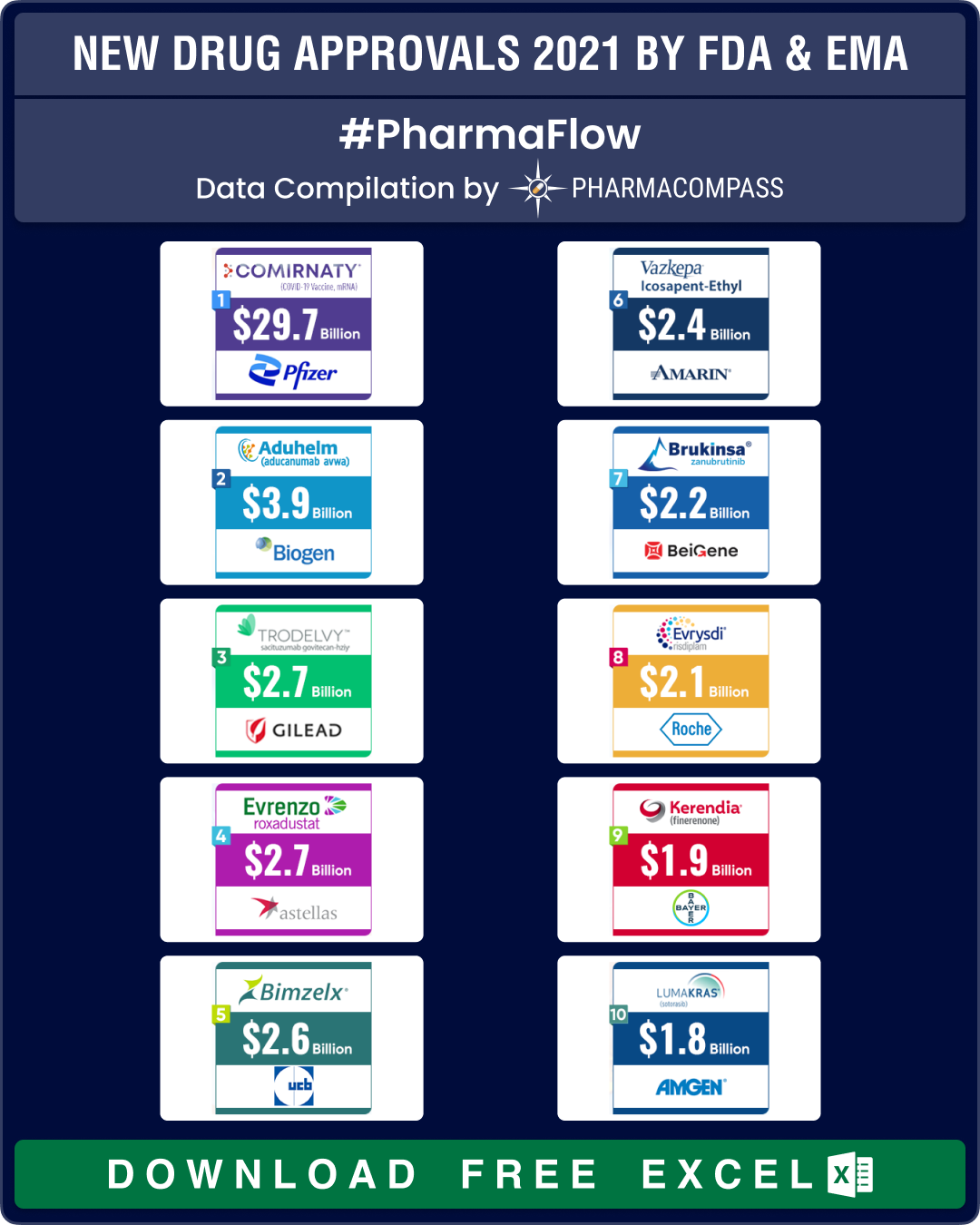
But the stars of 2021 were clearly the Covid vaccines. And amongst them, the Pfizer-BioNtech vaccine — Comirnaty (tozinameran) — stole the show.
Comirnaty has set new records for its speedy approval. BioNTech started work on this mRNA vaccine in January 2020, and partnered with Pfizer in March. In July 2020, the companies were holding phase 2/3 trials and provided safety and efficacy data to the FDA for an EUA, which came in by December 2020. In July 2021, the FDA granted priority review status to Pfizer-BioNTech’s application for a full approval, which came in just 18 months (in August 2021), when vaccine discovery and development, on an average, takes over 10 years.
Moderna’s mRNA-based Covid-19 vaccine —Spikevax (elasomeran) — also secured an EUA in December 2020. However, a full FDA approval is expected only by April 2022. These successes have driven a surge of investments in mRNA technology — for flu, other infectious diseases and cancer.
Commercially, Comirnaty has been a resounding success. As per our estimates, the vaccine should clock sales of US$ 29.7 billion in 2022. Similarly, Pfizer’s Paxlovid is likely to post sales of US$ 24.26 billion and Merck’s Molnupiravir should bring in sales of US$ 7
billion in 2022.
Among other drugs approved in 2021, we expect Lumakras to post sales of US$ 1.76 billion by 2024 and Biogen’s Aduhelm to post sales of US$ 3.9 billion by 2024.
View New Drug Approvals in 2021 with Estimated Sales (Free Excel Available)
Our view
During
2021, we saw drug companies work at breakneck speeds to discover Covid-19 drugs
and vaccines and regulatory agencies work against all odds to conduct
inspections, grant authorizations and approvals.
Given the inordinate circumstances, we saw some controversies too. For instance, FDA’s approval of Biogen’s Aduhelm divided the scientific community
and triggered multiple investigations into the FDA’s decision-making process. Even the speedy
approval of Comirnaty drew concerns from scientists, academics and doctors.
They recently won a Freedom of Information Act (FOIA) request, seeking data on the vaccine to
help them “offer solutions and address serious issues with the current vaccine program.” In September 2021, some FDA scientists had resigned over the issue of administering vaccine boosters and there was much debate
globally over whether booster doses of vaccines were needed or not.
The drug review process in the United States is recognized the world over as the gold standard, due to the rigorous evaluation processes for safety, quality, and effectiveness of therapies followed by the FDA. Therefore, these controversies do not augur well for the global pharmaceutical industry. We do hope that these are behind us in 2022, along with the Covid-19 pandemic, and that the drug industry begins to see a semblance of normalcy.
View New Drug Approvals in 2021 with Estimated Sales (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : New-Drug-Approvals-by-FDA-EMA-in-2021 by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








