By PharmaCompass
2019-12-31
Impressions: 14840
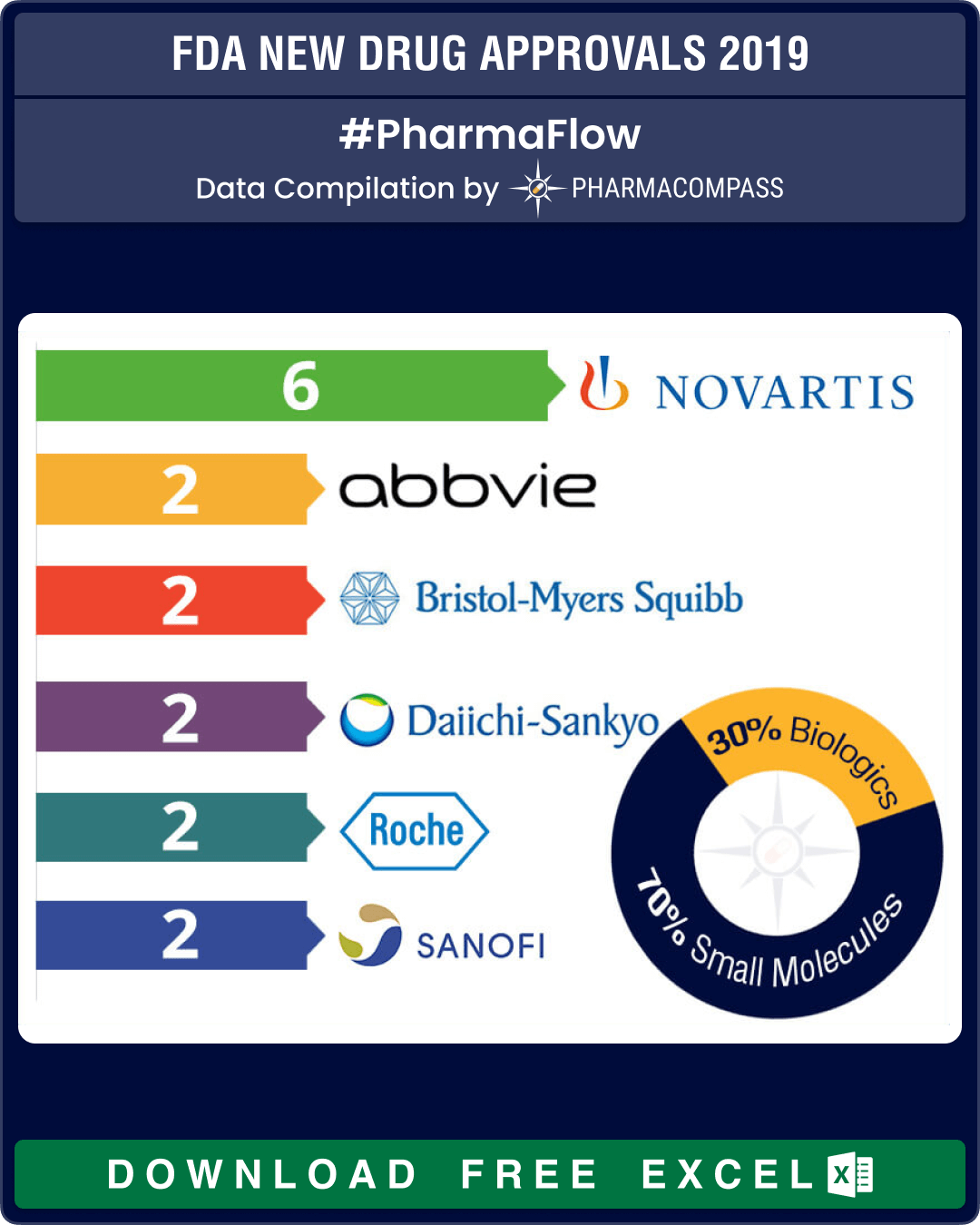
The US Food and Drug Administration’s (FDA) momentum of new drug approvals dropped marginally in 2019 — while 62 novel drugs were approved in 2018, 54 were approved in 2019. FDA’s Center for Drug Evaluation and Research (CDER) approved 48 drugs while another six were approved by the Center for Biologics Evaluation and Research (CBER).
Novartis dominated new drug approvals as CDER approved five of its new drugs across five different therapeutic areas. But then, it also made headlines when the FDA raised data accuracy issues over the Swiss drugmaker’s gene therapy — Zolgensma — which sent the company into a crisis management mode.
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
The gene therapy drug came into the fold of Novartis in April 2018 through the US$ 8.7 billion acquisition of Illinois-based AveXis Inc.
Zolgensma — the world’s most expensive drug — was approved as a one-time treatment for spinal muscular atrophy (SMA) in May 2019.
However, in March 2019, Novartis learnt of manipulation in the raw data derived from a mice assay used at the time to test Zolgensma samples, but only alerted the FDA in June — about a month after the drug gained US approval.
Post that, Novartis came under fire from US lawmakers who said the company should have informed the regulators about the data irregularities before the drug’s approval in May, instead of waiting to conclude an internal investigation.
Novartis’ other approvals were brolucizumab (for treatment of macular degeneration), siponimod (to treat multiple sclerosis), alpelisib (breast cancer), crizanlizumab (sickle cell disease) and triclabendazole (fascioliasis, a tropical disease).
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
Vertex’s Trikafta leads in estimated sales potential
At PharmaCompass, we compiled the sales forecasts for the new drugs approved by the FDA in 2019. Vertex’s cystic fibrosis treatment — Trikafta — with expected sales of US$ 3.935 billion by 2024, leads our list.
Trikafta is a combination of ivacaftor, tezacaftor and elexacaftor. Vertex already has three cystic fibrosis drugs on the market — Kalydeco (a standalone ivacaftor treatment), Symdeko (a combination of ivacaftor and tezacaftor) and Orkambi (which contains ivacaftor and lumacaftor).
Trikafta’s stellar clinical data made the FDA approve the drug within three months of Vertex’s application filing and five months before FDA’s action date.
Abbvie’s Humira, the top-selling drug in the world, is beginning to face generic competition in certain parts of the world and two new drug approvals in 2019 — Skyrizi (risankizumab for plaque psoriasis) and Rinvoq (upadacitinib for rheumatoid arthritis) — are critical to AbbVie's plans to grow beyond Humira. The two drugs are expected to contribute over US$ 5.5 billion by 2024 to AbbVie’s top-line.
In April, AstraZeneca struck a deal worth US$ 6.9 billion with Japanese drug major Daiichi Sankyo, to jointly develop and commercialize the antibody drug conjugate cancer therapy —trastuzumab deruxtecan. The breast cancer treatment won FDA approval in December and analysts forecast sales of US$ 2.5 billion by 2024 with some believing that the drug could achieve US$ 7 billion in peak sales.
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
The rise of new drugs from Asia
While Daiichi won the FDA nod for its cancer treatment — Turalio (pexidartinib) — new drugs from established Japanese drug makers like Shionogi, Kyowa Kirin and Eisai also bagged the American agency’s approval.
Shionogi’s novel antibiotic — Fetroja (cefiderocol) — got approved for complicated urinary tract infections while Kyowa won the nod for its Parkinson’s treatment — Nourianz (istradefylline). Eisai’s insomnia drug — Dayvigo (lemborexant) — was one of the last drugs approved in the year and is expected to generate sales of US$ 329 million.
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
In 2019, the FDA also approved new drugs from China and Korea. Zanubrutinib (branded as Brukinsa) developed by Chinese biotech BeiGene won accelerated approval for adult patients with mantle cell lymphoma (MCL) — a typically aggressive and rare form of blood cancer.
This was followed, almost immediately, by news that the agency approved a new treatment for partial-onset seizures in adults. The drug — cenobamate — will be sold as Xcopri by SK Life Science, the US subsidiary of the massive Korean conglomerate SK Group. Xcopri will provide another therapeutic option to patients with epilepsy. This approval marked the first time a Korean company independently took a compound from the stage of discovery to FDA approval.
Xcopri is SK Life Science’s first commercial product and wraps a decade of quiet growth from SK Life Science, which has been developing its presence in the pharmaceutical industry.
The company plans to hire a salesforce to support the drug’s launch, which is expected to generate over US$ 1.5 billion in sales.
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
Sanofi’s controversial dengue vaccine, cast-off drug get approved
The FDA very narrowly approved Sanofi’s controversial dengue vaccine — Dengvaxia. The vaccine, which took 20 years to develop, got embroiled in a controversy when in 2017, Sanofi disclosed that Dengvaxia could increase the risk of severe dengue in children who had never been exposed to the virus.
In Philippines, 800,000 school-age children had been vaccinated against dengue, the world’s fastest growing infectious disease.
The year 2019 had begun with Bristol-Myers Squibb (BMS) and Celgene announcing their US$ 74 billion mega-merger. As the firms worked through the year to get the merger approved, the FDA signed off on Celgene’s myelofibrosis drug Inrebic (fedratinib).
Inrebic — the once-daily oral drug — is the first new treatment in nearly a decade approved to treat myelofibrosis. The story of Inrebic’s rise from the dead is a major success for Celgene, which picked up the drug as a part of a US$ 1.1 billion acquisition of Impact Biomedicines in January 2018. Before that, Inrebic had passed through multiple hands.
Fedratinib was a flop for Sanofi as patients began to develop a dangerous neurological condition tied to vitamin B deficiency called Wernicke’s encephalopathy. As a result, the FDA put a clinical hold on it in 2013 and Sanofi ultimately shelved the effort.
Executives at BMS have said the drug is among a group of potential blockbusters at Celgene.
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
Our view
With over 40 companies from across the world getting new drugs approved in 2019 across 17 therapeutic areas, the horizon for new drug development across the globe continues to widen.
Although 2018 was a landmark year for approvals, given all the development activity underway, the day is not far when this record too will get beaten. And 2020 could well witness that day!
View FDA’s New Drug Approvals in 2019 with estimated sales (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : FDA New Drug Approvals in 2019 by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








