Chinese FDA-registered generic facilities gain steam, India maintains lead with 396 facilities
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect
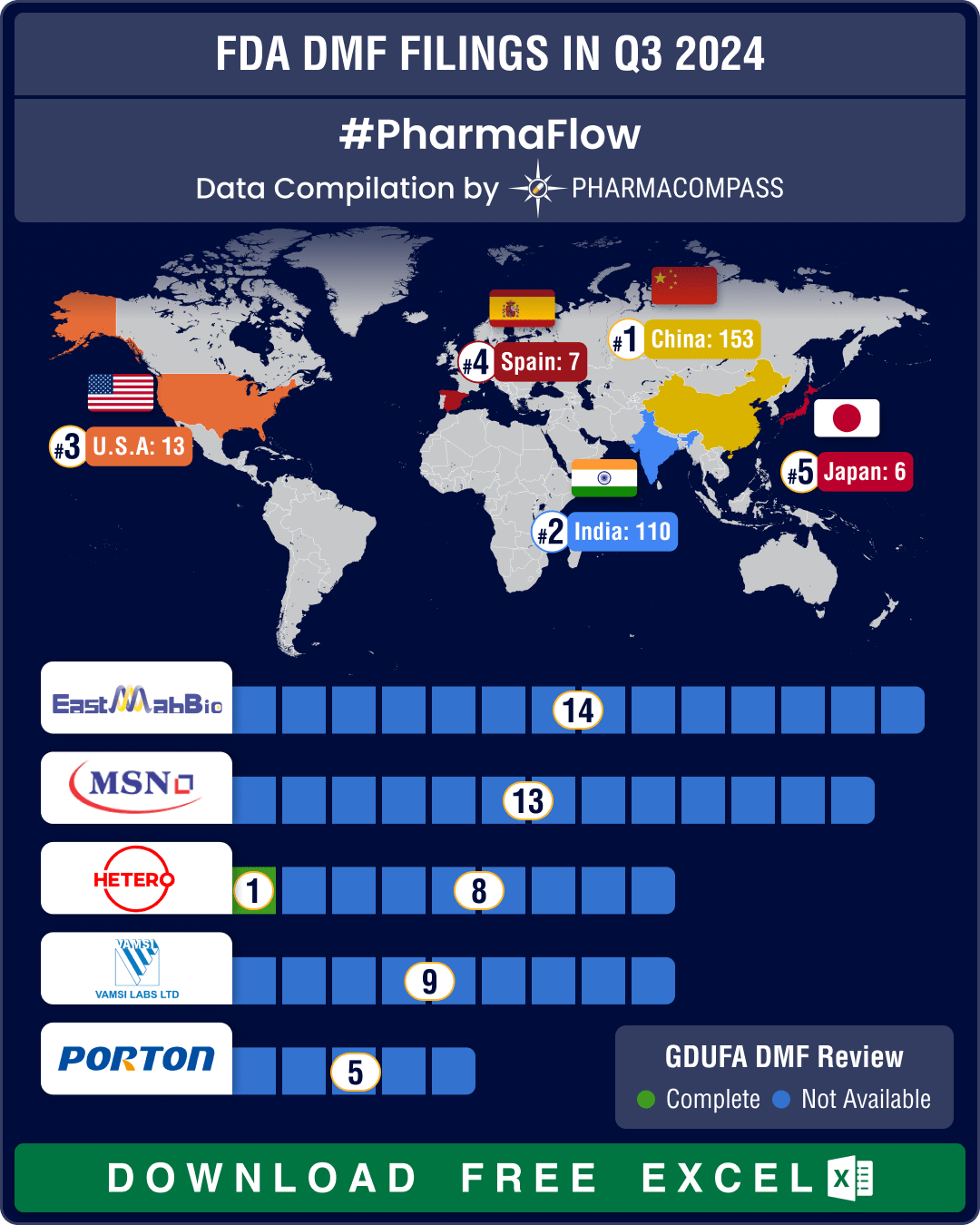
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica


 Market Place
Market Place Sourcing Support
Sourcing Support