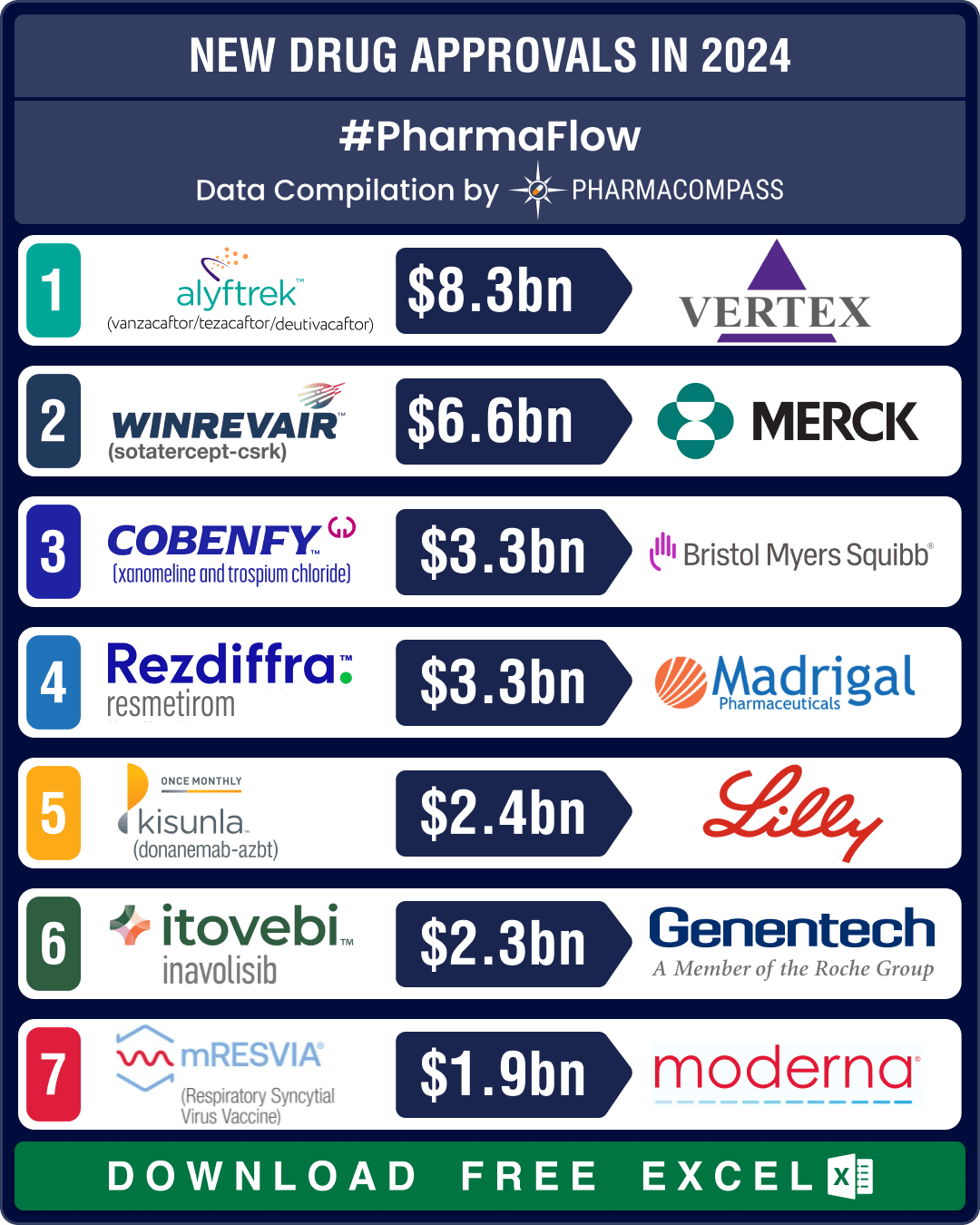
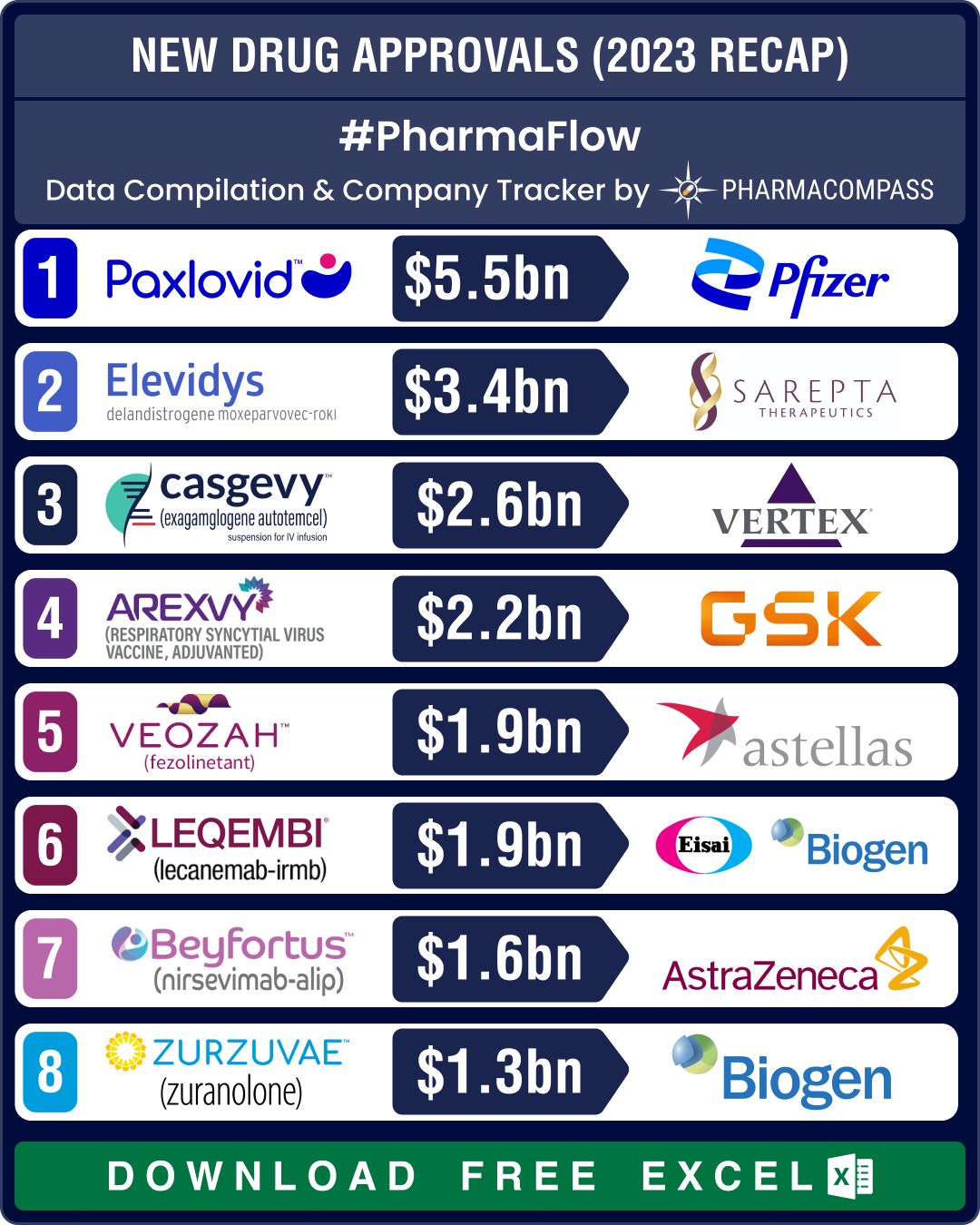
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
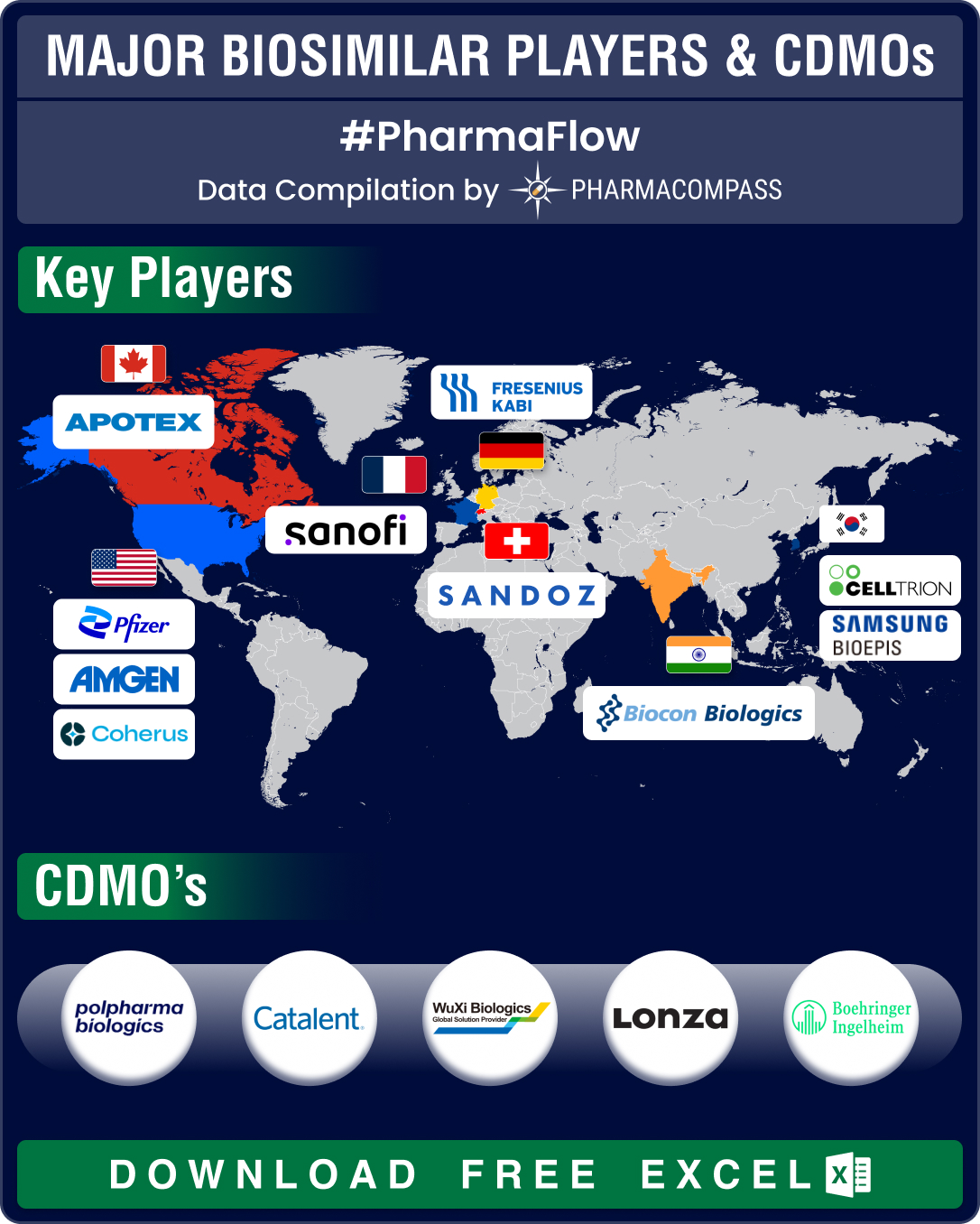
FDA approves record eight biosimilars in H1 2024; okays first interchangeable biosimilars for Eylea
Biologics, or complex drugs that are derived from living organisms, have revolutionized treatment of


 Market Place
Market Place Sourcing Support
Sourcing Support