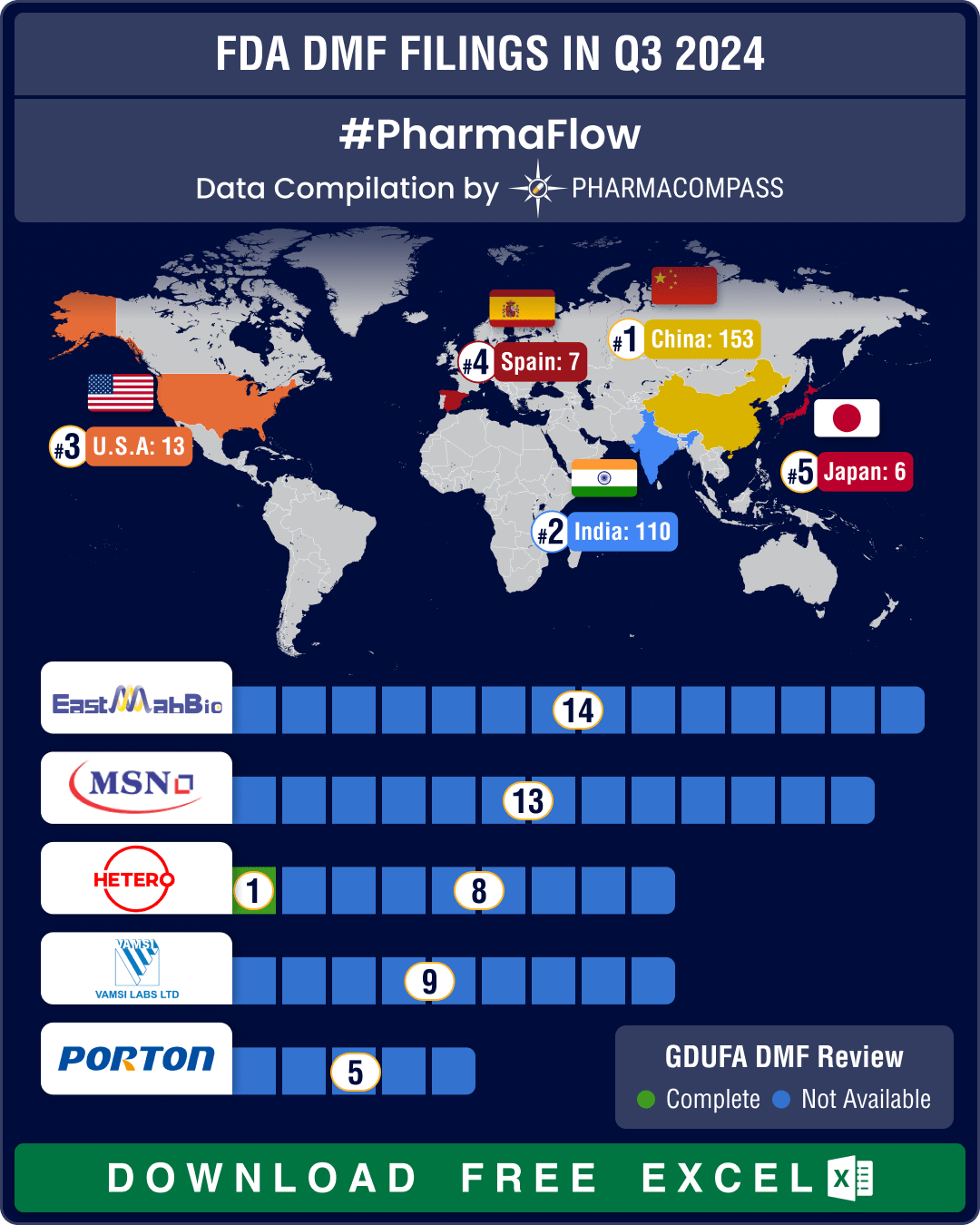
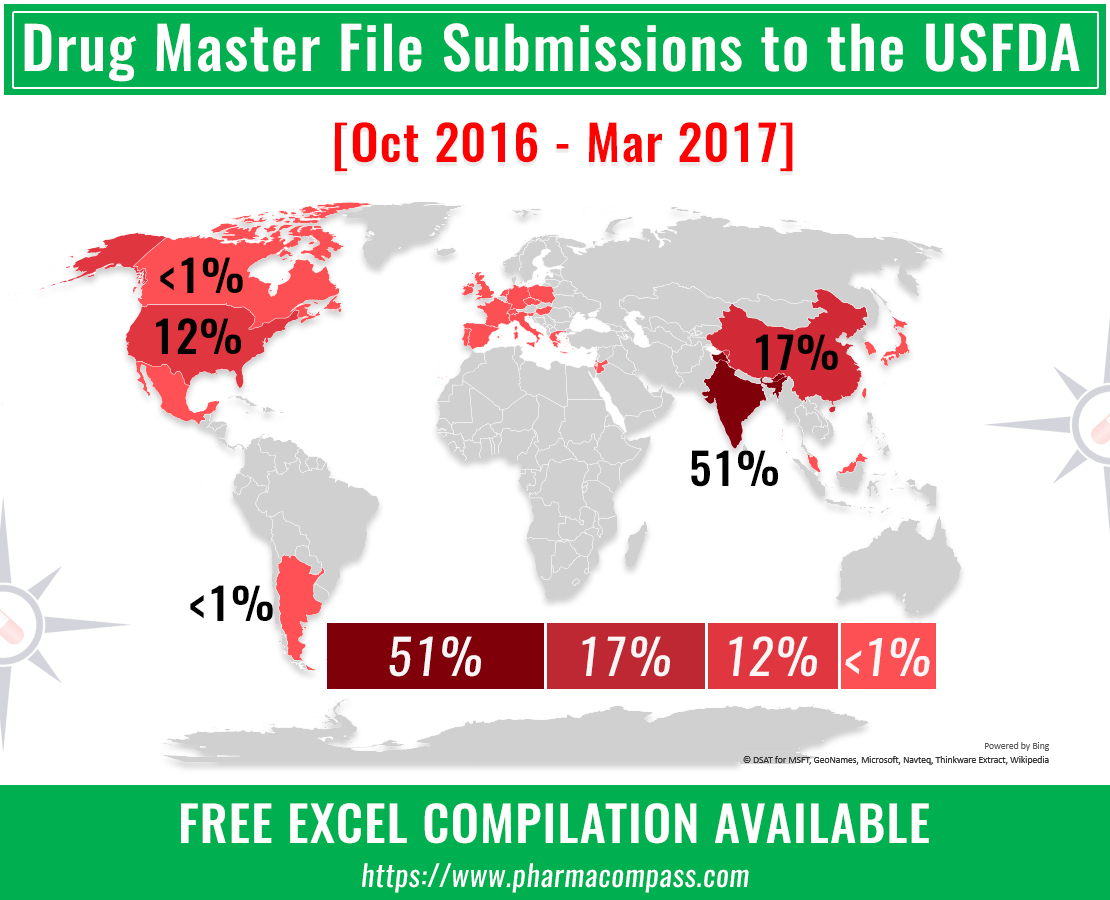
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica
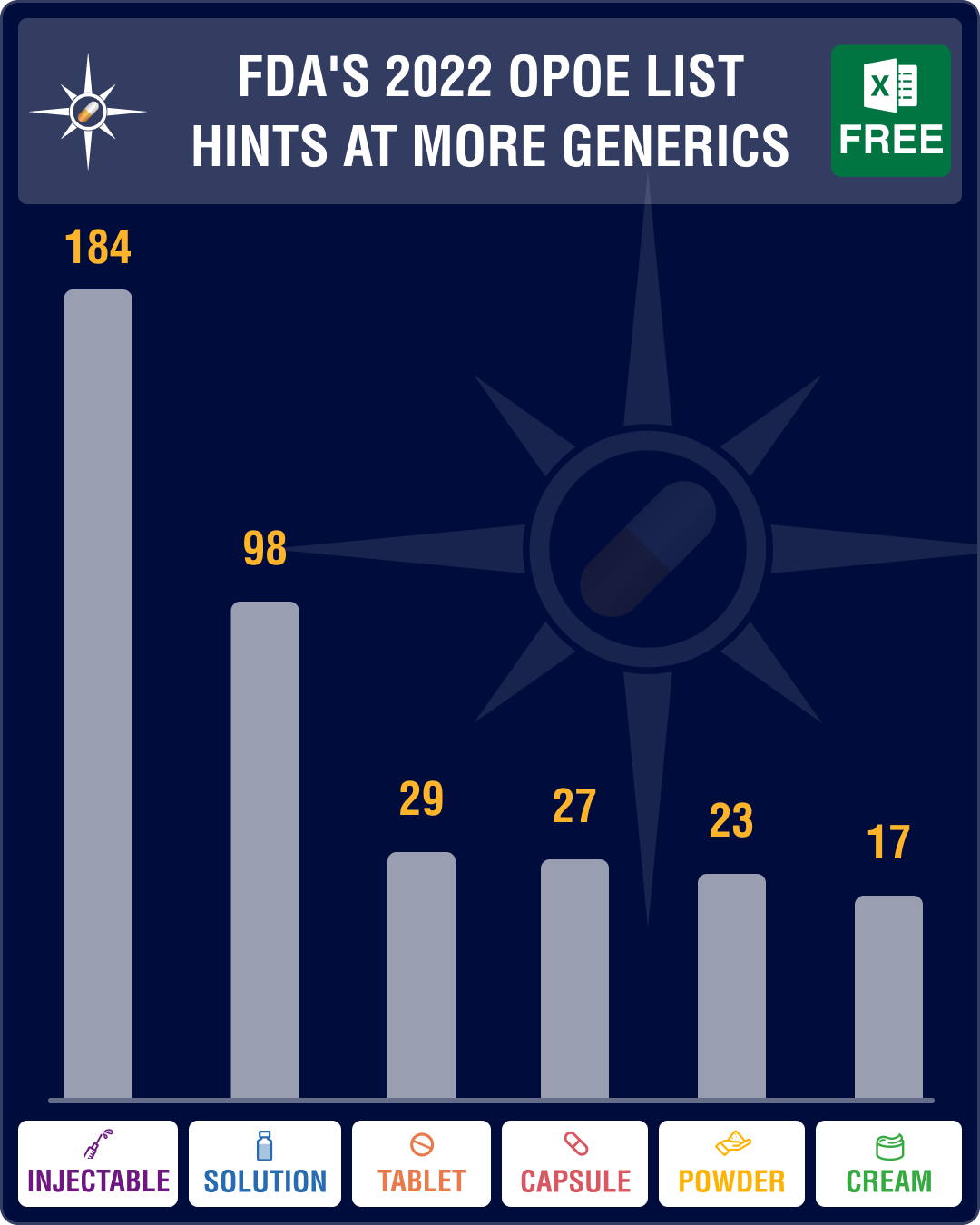
FDA’s list of off-patent drugs suggests higher approvals of first generics in 2022
We usher in 2023 with the key highlights of the US Food and Drug Administration’s December 202
Top drugs and pharma companies by sales in 2020
Last
year, the pandemic impacted everyone’s life in one way or the other. It turned
the lime


 Market Place
Market Place Sourcing Support
Sourcing Support