Excipient Market Overview: Evonik launches high-purity excipients; India mandates disclosures from March 2026
The global pharmaceutical excipients market continued to evolve in the third quarter (Q3) of 2025, s
CDMO Activity Tracker: Veranova, Carbogen lead ADC investments; Axplora, Polfa Tarchomin, Famar expand European footprint
During the second quarter (Q2) of 2025, contract development and manufacturing organizations (CDMOs)
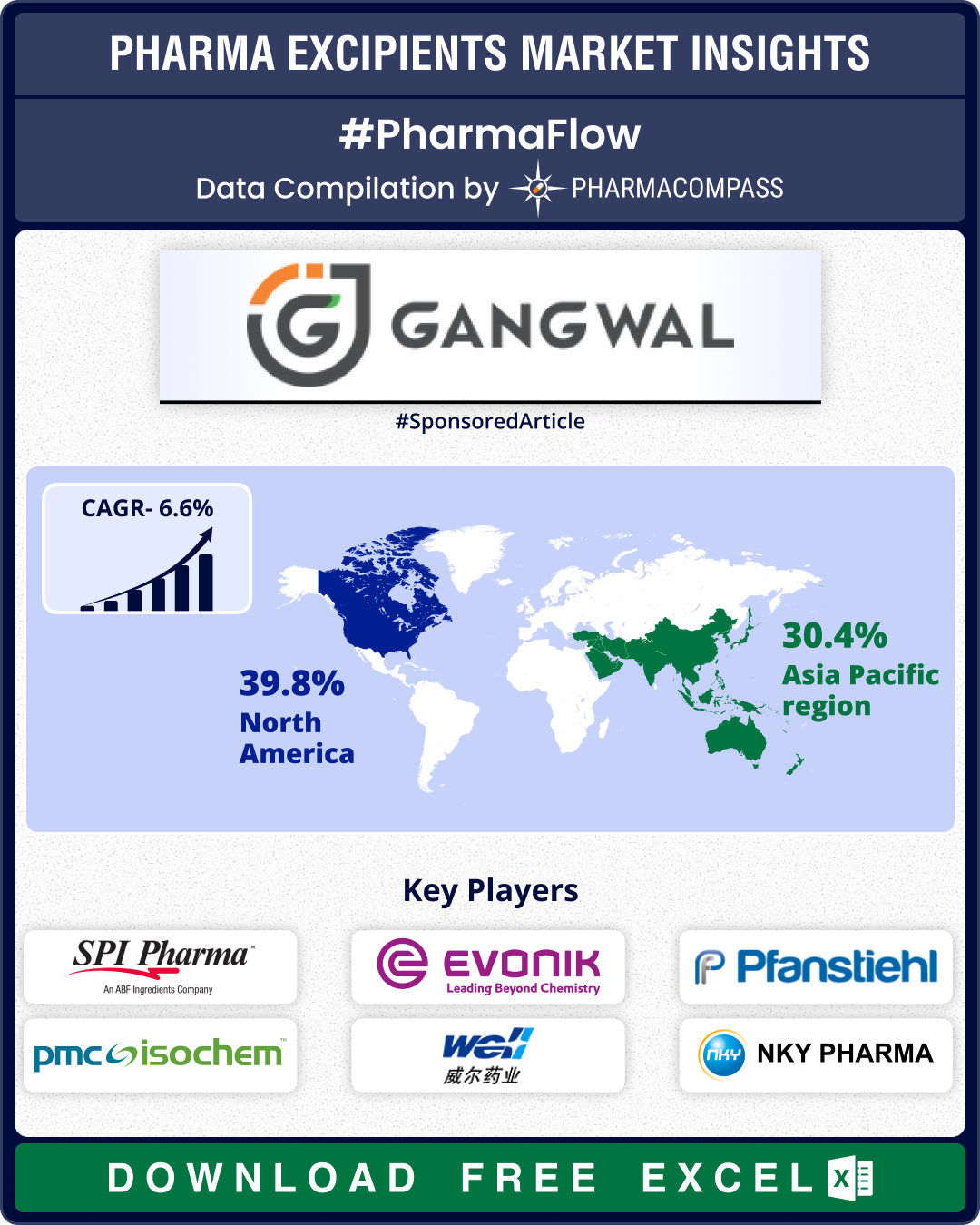
Excipient Market Overview: Roquette announces restructuring post IFF Pharma buyout; WHO, FDA advance regulatory frameworks
The pharmaceutical excipients market saw significant strategic
consolidations, technological develo
CDMO Activity Tracker: Bora, PolPharma make acquisitions; Evonik, EUROAPI, Porton announce technological expansions
The contract development and
manufacturing organization (CDMO) space continued to grow at an impres
Chinese FDA-registered generic facilities gain steam, India maintains lead with 396 facilities
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect
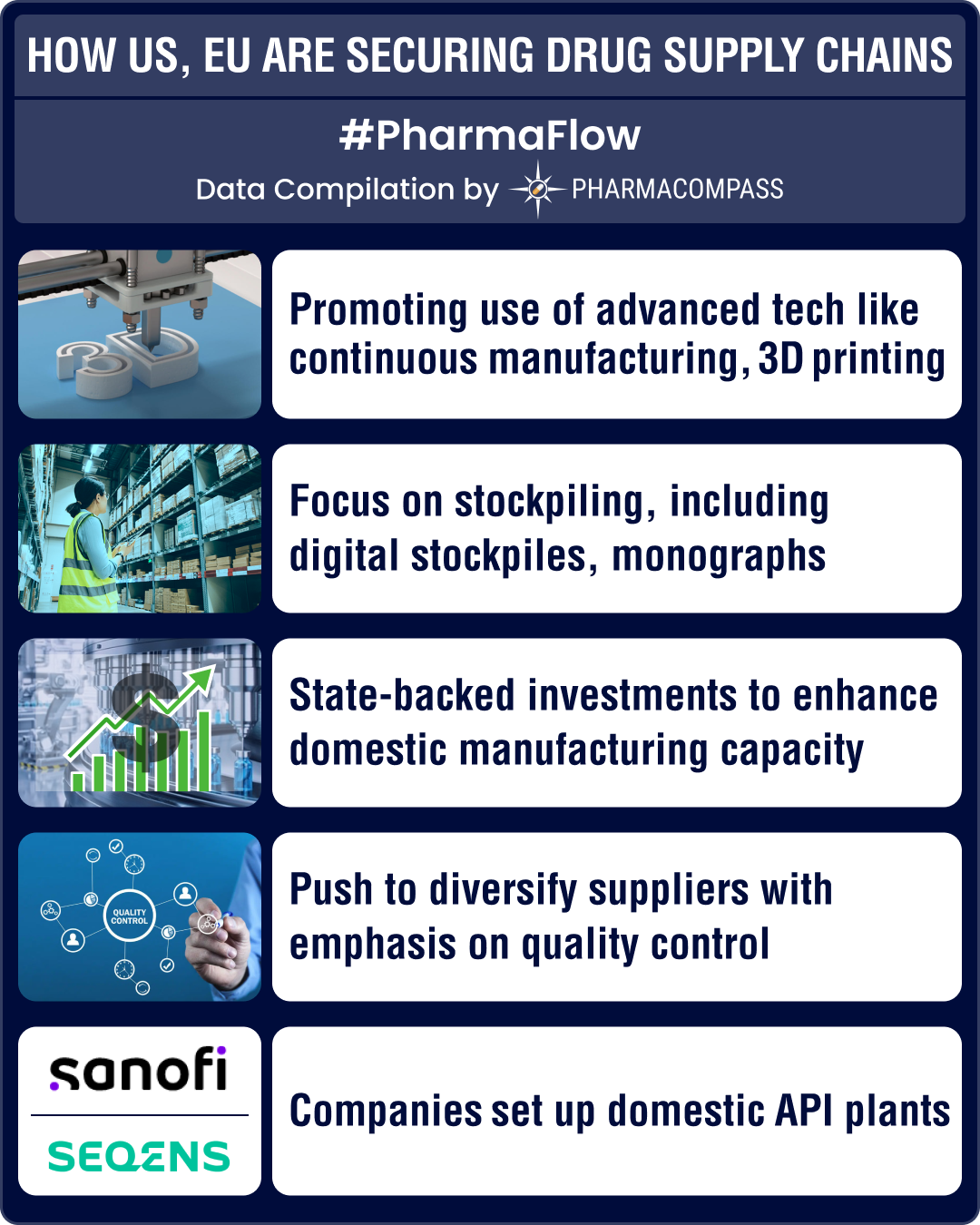
US, Europe turn to advanced manufacturing, stockpiling to strengthen drug supply chains
Over the last few decades, the United States and Europe have saved trillions of dollars by importing
Excipient Market Overview: Roquette, Seqens, Evonik make strategic moves; new guidelines deal with contamination
The pharmaceutical industry has long recognized the critical role
excipients or inactive ingredient


 Market Place
Market Place Sourcing Support
Sourcing Support