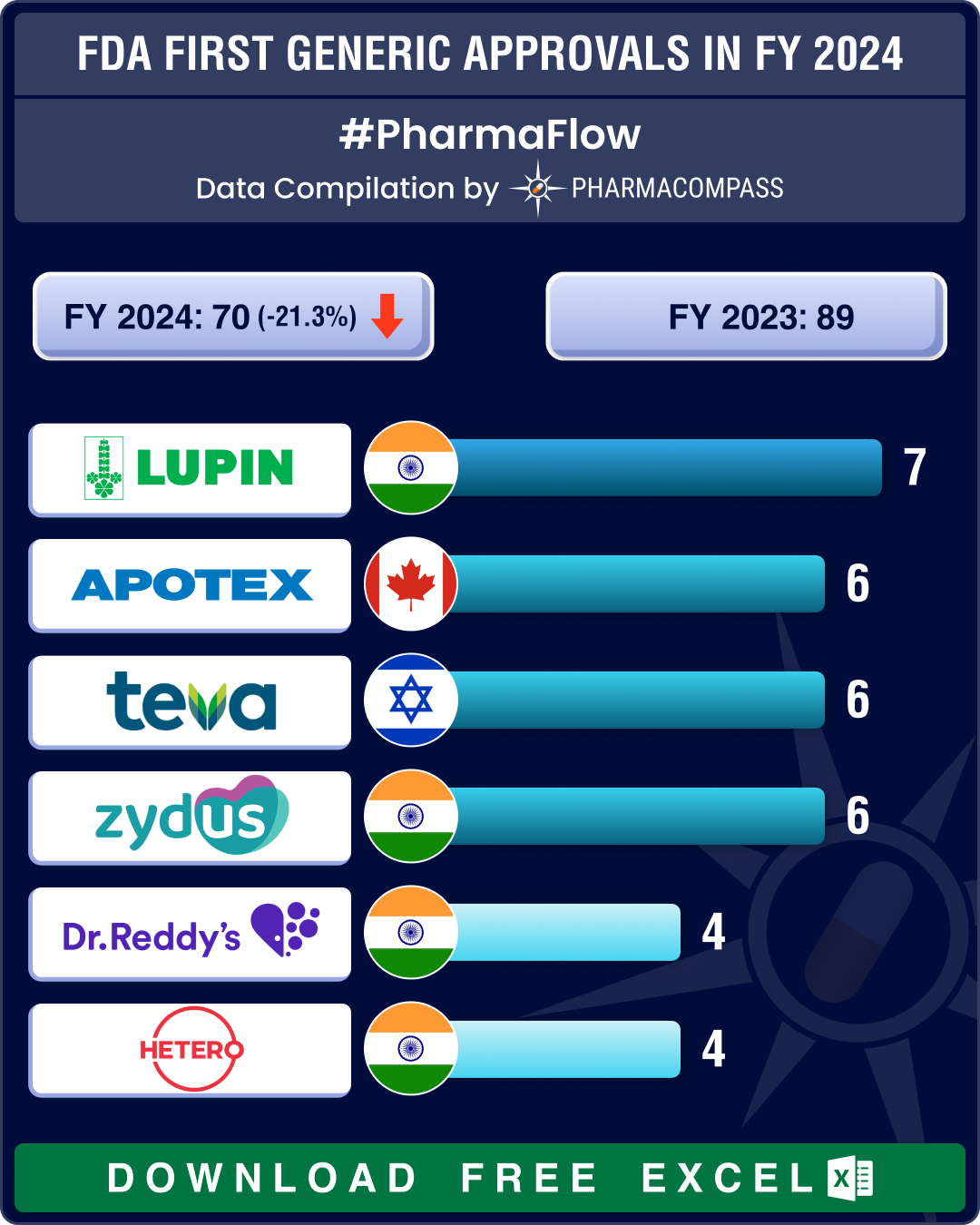
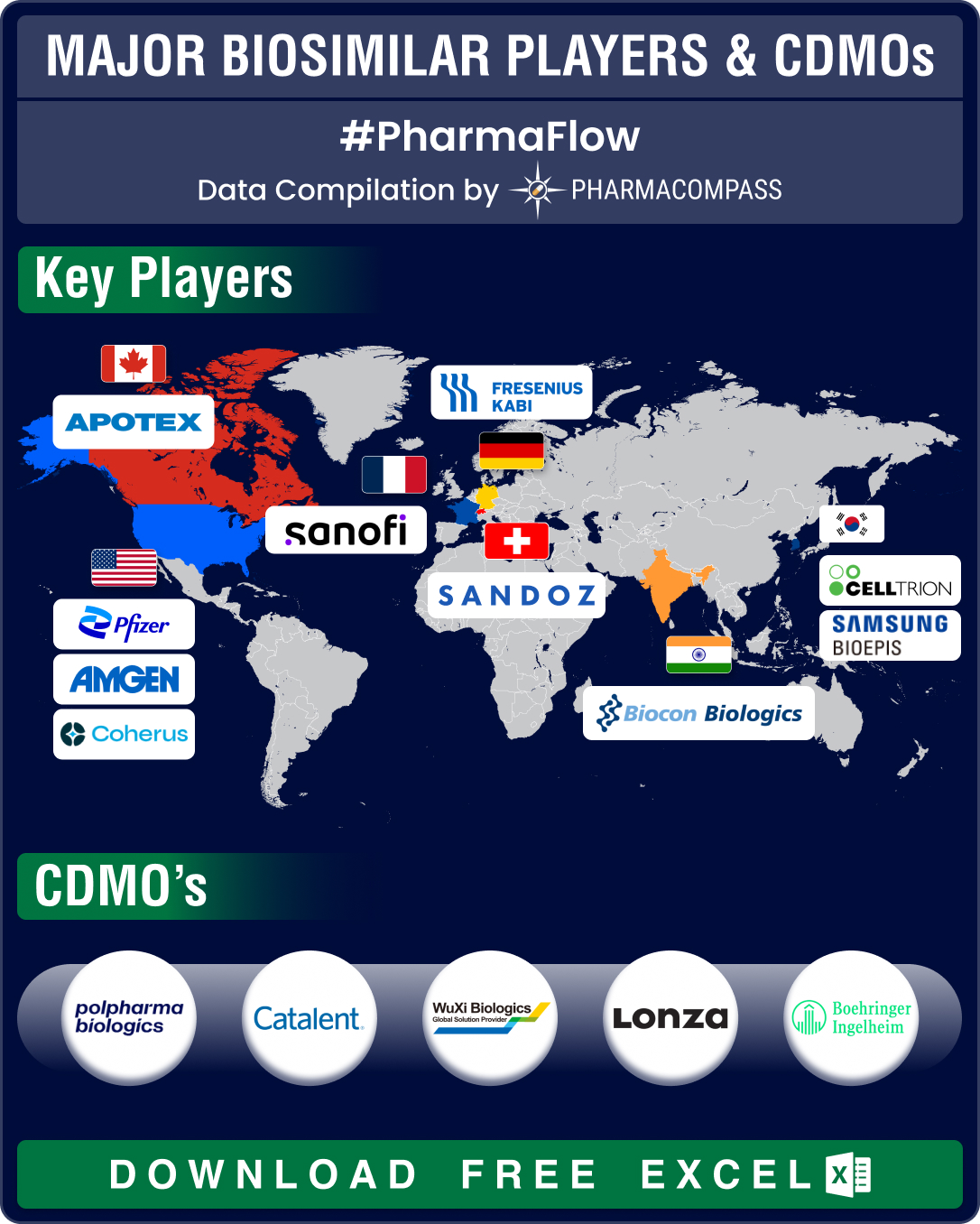
FDA’s first generic approvals slump 21% in 2024; Novartis’ top seller Entresto, cancer blockbuster Tasigna lead 2024 patent cliff
A watershed moment in the journey of a drug is when it transitions from being a patented, high‐
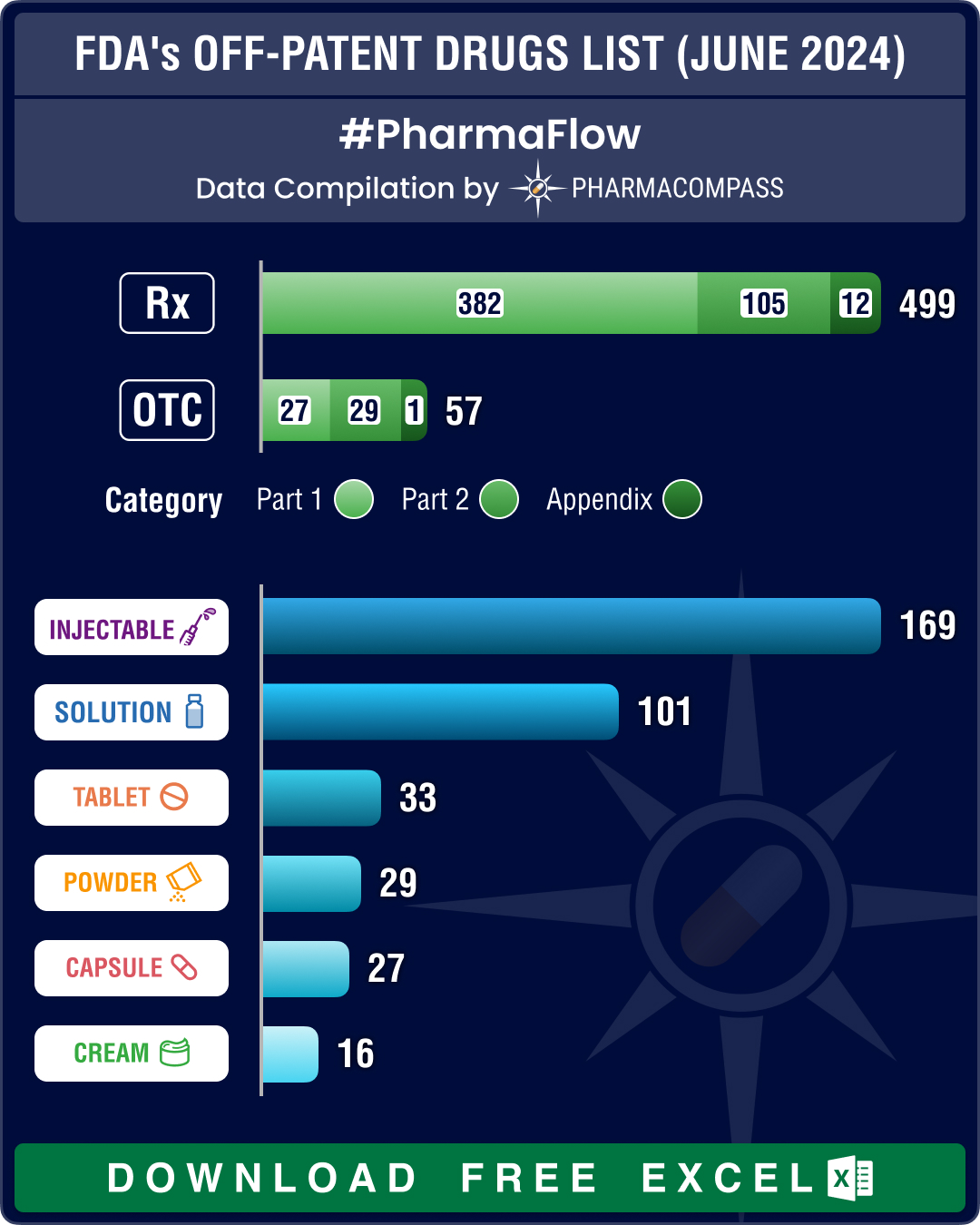
FDA’s June 2024 list of off-patent, off-exclusivity drugs sees rise in cancer, HIV treatments
This week PharmaCompass brings to you key highlights of the US Food and Drug Administration’s


 Market Place
Market Place Sourcing Support
Sourcing Support