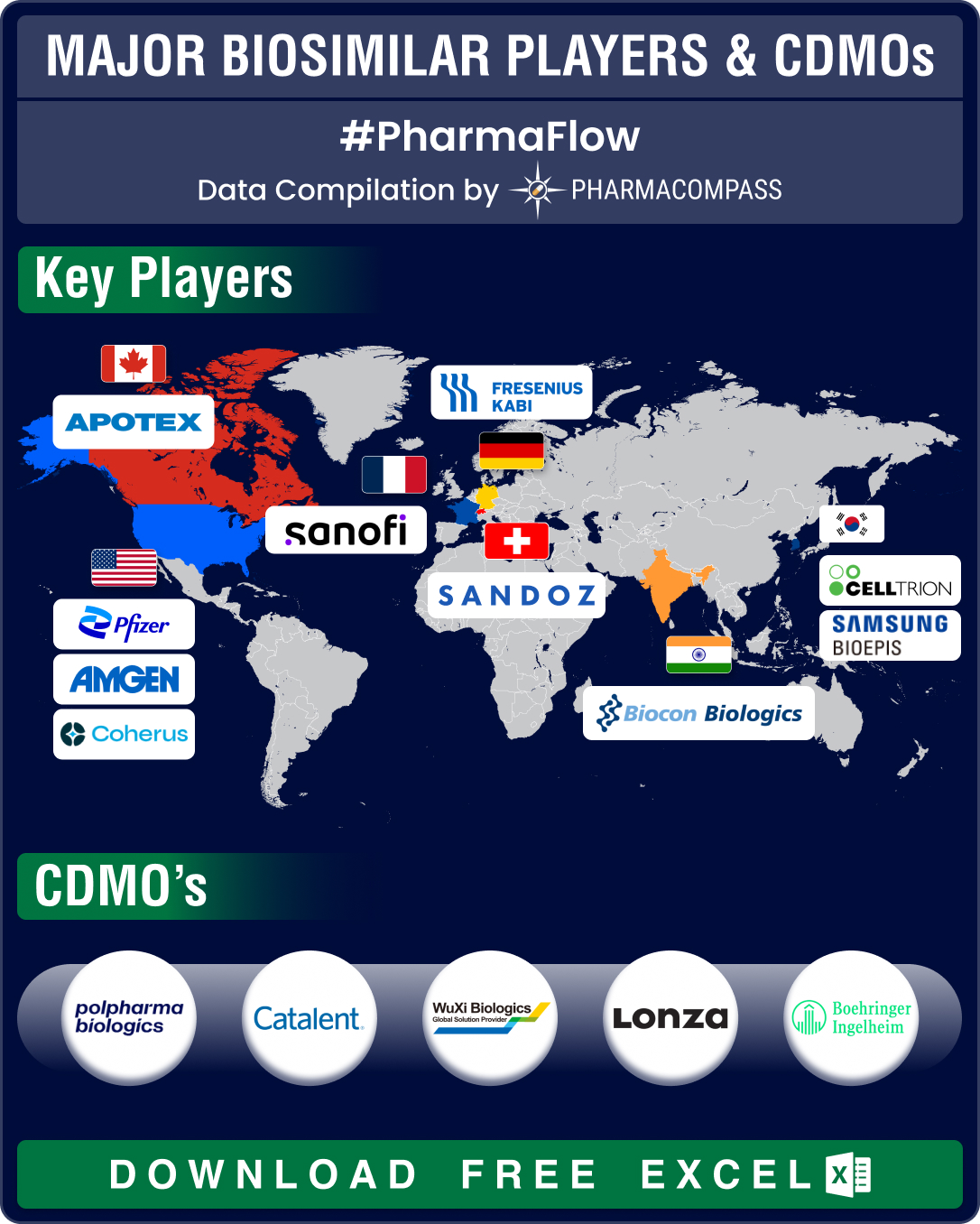
Biologics, or complex drugs that are derived from living organisms, have revolutionized treatment of various conditions such as cancer, autoimmune diseases, and chronic illnesses. In 2023, eight out of 10 of the world’s top-selling drugs were biologics, including Merck’s Keytruda, AbbVie’s Humira, and Sanofi’s Dupixent.Due to their high costs, accessibility of biologics has been a challenge. That’s why biosimilars, or game-changing copycats of biologics that provide highly similar yet more affordable alternatives to established biologics, are becoming popular.The first biosimilar — Sandoz’ Zarxio — was approved by the US Food and Drug Administration (FDA) in 2015. Its reference biologic was Amgen’s Neupogen (filgrastim). Since then, the global market for biosimilars has been growing at an impressive pace — between 2015 and 2020, it grew at a whopping compounded annual growth rate (CAGR) of 78 percent, touching US$ 17.9 billion in size. It is expected to continue growing at a CAGR of 15 percent and reach a size of about US$ 75 billion by 2030.Major
biosimilar players include Amgen, Sandoz, Samsung Bioepis, Pfizer, Biocon Biologics, Celltrion, Stada Arzneimittel, Accord Healthcare, Fresenius Kabi, Coherus Biosciences, Apotex, and Sanofi. The increasing demand for
biosimilars has propelled growth in contract manufacturing. Some of the
leading contract manufacturing organizations (CMOs) and contract development
and manufacturing organizations (CDMOs) that manufacture biosimilars are Polpharma Biologics, Catalent, Pfizer CentreOne, Lonza, Boehringer Ingelheim BioXcellence, Thermo Fisher Scientific, WuXi Biologics, and FUJIFILM Diosynth
Biotechnologies.Access the Interactive Dashboard for Biosimilar Developments (Free Excel)Amgen, Sandoz top list of ‘approved biosimilars’; FDA okays 8 copycats in H1 2024Over
the recent years, regulatory agencies like the FDA
and the European Medicines Agency (EMA) have established rigorous approval pathways for biosimilars.Since 2015, FDA has
approved 53 biosimilars, while the EMA has approved 86 biosimilars. Among the US, European and
Canadian markets, Amgen and Sandoz are tied in the first place
with 13 approved biosimilars each. Samsung Biologics has nine approved
biosimilars, followed by Pfizer with eight and Biocon Biologics with seven. In the first half of this year, FDA set a record by approving eight biosimilars — the highest for H1 of any year. EMA has okayed six biosimilars so far in 2024.In 2023, five biosimilars were approved by the FDA with just one
being okayed in the first half. The year marked the end of exclusivity for Humira after 20 years, in which it
netted a total of US$ 200 billion in sales. AbbVie’s flagship autoimmune drug has a record 10 biosimilars.Johnson & Johnson’s Stelara also lost exclusivity in 2023 and as many as 11
drugmakers hope to bring its biosimilars to the market. Amgen’s Wezlana was the first biosimilar to
Stelara, and it was approved as interchangeable by FDA in October last year.Access the Interactive Dashboard for Biosimilar Developments (Free Excel) FDA approves first
interchangeable biosimilar for Eylea, cuts regulatory feeDeveloping a biosimilar costs both money and time. According to
Pfizer, developing a biosimilar can take five to nine years and cost over US$ 100 million, not including regulatory fees.In October 2023, FDA slashed its fees with the program fee at US$ 177,397, down from US$ 304,162. The application fees for products that require clinical data has been set at US$ 1,018,753, down from US$ 1,746,745. The application fee for products that don’t require clinical data has been set lower — at US$ 509,377 — down from US$ 873,373 set earlier. This reduction in application fee has propelled demand for contract manufacturing of biosimilars.There has also been a rise in approvals of interchangeable
biosimilars this year. Interchangeable biosimilars meet additional requirements
and may be substituted for its reference product by a pharmacist without
consulting the prescriber. This year saw FDA approve the first interchangeable biosimilars for bone cancer
drug denosumab (Prolia and Xgeva) in
Jubbonti and Wyost as well as for eculizumab (Soliris) in Bkemv.In May, FDA approved the first interchangeable biosimilars
for eye drug aflibercept (Eylea) in Opuviz and
Yesafili. Other biosimilars approved in 2024 include Simlandi for adalimumab (Humira), Tyenne for tocilizumab (Actemra), Selarsdi for ustekinumab (Stelara), and Hercessi for trastuzumab (Herceptin).Access the Interactive Dashboard for Biosimilar Developments (Free Excel) Merck’s Keytruda, BMS’ Opdivo, Novartis’ Cosentyx brace for biosimilar competitionHealthcare spending in the US is projected to rise from US$ 4.5
trillion in 2022 to US$ 6 trillion by 2027. While biologics involve just two
percent of prescriptions, they account for 46 percent of all pharmaceutical
spending. In 2022, US$ 252 billion was spent on biologics.Biosimilar-related savings in 2023 were estimated to be US$ 9.4
billion in the US and € 10 billion (US$ 10.68 billion) in Europe. With expensive and widely used drugs like AbbVie’s Humira, J&J’s Stelara, and Regeneron’s Eylea coming under competition, US
savings are projected to reach US$ 181 billion through 2027. Between 2026
and 2032, about 39 blockbusters are set to lose exclusivity in the US and Europe. Merck’s Keytruda (pembrolizumab) was the world’s top-selling drug last year, generating US$ 25 billion in sales. Its patent is set to expire in 2028 with sales expected to drop
19 percent to US$ 27.4 billion in 2029 from US$ 33.7 billion the previous year. Samsung
Bioepis and Amgen initiated phase 3 trials of pembrolizumab in April
and May of this year, respectively.Opdivo (nivolumab), belonging to the same
class of drugs, competes with Keytruda and is also set to lose patent
protection in 2028. It hauled in US$ 10 billion in total global sales in 2023
for Bristol Myers Squibb. The key patents of Novartis’ Cosentyx (secukinumab) are set to expire between
2025 and 2026. Cosentyx saw sales of US$ 5 billion in 2023. Taizhou Mabtech Pharmaceutical and Bio-Thera Solutions are conducting phase 3 trials of secukinumab.Access the Interactive Dashboard for Biosimilar Developments (Free Excel) Our viewWith
over 2 billion people worldwide unable to access life-saving medicines,
biosimilars hold the key to healthcare accessibility. In 2023, a record 13 biosimilars were launched in the market — the highest for a single year. And this included nine much-anticipated biosimilars to AbbVie’s Humira. In April this year, FDA announced a Biosimilars Action Plan to streamline the development of biosimilars. With a sharp focus on biosimilars, we expect more records to be broken in the near term. New launches of biosimilars to drugs like J&J’s Stelara, Regeneron’s Eylea and Merck’s Keytruda will surely help in creating new records.
Impressions: 1385
In the intricate world of molecular biology, oligonucleotides stand out as versatile, powerful molecules. Oligonucleotides are essentially short, single strands of DNA or RNA that modulate gene expression. There are various oligonucleotide therapy (ONT) agents (such as antisense, deoxyribozymes, siRNA and CRISPR/Cas) that offer promising therapeutic tools.A variation of gene therapy, oligonucleotide gene therapies (OGTs) are manufactured using synthetic oligonucleotides. These therapies are designed to enter cells. ONTs act like tools that can fine-tune the behavior of certain genetic instructions, and are therefore often designed to treat rare and genetic diseases and cancers. Sometimes, they may be delivered into cells through lipid nanoparticles or adeno-associated viruses (AAV), halting the translation of a specific protein. Oligonucleotides have also revolutionized vaccine development through the creation of nucleic acid vaccines, such as mRNA vaccines.The first oligonucleotide
drug, known as fomivirsen, was approved by the
US Food and Drug Administration (FDA) back in 1998. It was developed by Ionis Pharmaceuticals (then known as Isis Pharmaceuticals), and was approved to treat a rare eye disease. However, ONT approvals have picked up since 2016. Currently, there are 20 oligonucleotide drugs approved by the FDA and the European Medicines
Agency (EMA) and a majority of them treat orphan and rare
diseases.In 2023, FDA approved four ONTs — AstraZeneca-Ionis’ Wainua (eplontersen), Novo Nordisk owned Dicerna Pharmaceuticals’ Rivfloza (nedosiran), Biogen-Ionis’ Qalsody (tofersen) and Iveric Bio’s Izervay (avacincaptad pegol).In 2021, the global ONT market size was estimated to be US$ 18.2 billion. It is expected to increase to US$ 51.4 billion by 2029, growing at a compounded annual rate (CAGR) of 13.85 percent. Similarly, the market for oligonucleotides synthesis (or the process of producing oligonucleotides) was estimated at US$ 7.7 billion globally in 2022
and is expected to grow 11.8 percent CAGR to reach US$ 16.4
billion by 2030. Some of the bigger players in oligonucleotides synthesis are Danaher Corporation, Thermo Fisher Scientific, Merck KGaA, Eurofins, Agilent, Bio-Synthesis, EUROAPI, Eurogentec, STA Pharma, and Bachem.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available)ONTs address neuro disorders; four ONTs bring in US$ 1.24 bn for AlnylamONTs are widely applied to treat
neurodegenerative diseases, such as Duchenne muscular dystrophy (DMD). Multiple ONTs have been approved in Europe and the US for
DMD, such as Exondys 51 (eteplirsen), Vyondys 53 (golodirsen), and Amondys 45
(casimersen) from Sarepta and NS Pharma’s Viltepso (viltolarsen). Last year, Ionis bagged two approvals in the
space. FDA
approved its Biogen-partnered
therapy Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis
(ALS). The agency
also approved AstraZeneca and Ionis’ drug Wainua (eplontersen), rendering it as the
first self-administered treatment for a rare nerve damage
disease, known as hereditary transthyretin amyloidosis (ATTR-PN). Analysts
estimate global peak sales for Wainua to come in at US$ 750
million for ATTR-PN alone. The drug is also being
tested for transthyretin-mediated amyloid cardiomyopathy (ATTR-CM), a rare
heart muscle disorder.Alnylam
Pharmaceuticals has also successfully brought four ONTs to market in recent years — Onpattro (patisiran) and
Amvuttra (vutrisiran) for rare
nerve diseases, and Givlaari (givosiran) and Oxlumo (lumasiran). Givlaari has been approved to treat acute hepatic porphyria (a liver enzyme deficiency) while Oxlumo treats primary hyperoxaluria (a disorder characterized by increased urinary oxalate excretion). The four ONTs brought in US$ 1.24
billion for Alnylam in 2023.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Novartis
buys rights to siRNA therapy, GSK bets big on ONT pipeline through dealsAfter Alnylam discovered
inclisiran (Leqvio), Novartis acquired
global rights to the therapy in a US$ 9.7
billion deal. Leqvio was the first
FDA-approved small interfering RNA (siRNA) therapy
for LDL-C (bad cholesterol) reduction. It brought in US$ 355
million for Novartis in 2023.GSK has increasingly
been investing
in its ONT pipeline. The British
pharma has promised over US$ 5 billion in upfront and milestone payments in
multiple deals. In February, GSK exercised
its option to license Elsie Biotechnologies’ discovery platform to find
and develop novel ONTs. GSK has also entered a discovery collaboration with Wave Life Sciences.Last July, Japanese drugmaker Astellas Pharma completed its
approximately US$ 5.9 billion buyout of New
Jersey-headquartered Iveric Bio. Iveric focuses on retinal diseases and the deal gives Astellas drug candidates to treat about 160 million people with eye ailments. Subsequently, in August, Iveric’s Izervay (avacincaptad pegol) was approved by the FDA as a new
treatment for geographic atrophy (GA) secondary to age-related macular
degeneration (AMD).After Novo Nordisk acquired RNAi technology company Dicerna Pharmaceuticals for US$ 3.3 billion in 2021, the latter’s once-monthly RNAi therapy Rivfloza (nedosiran) saw FDA approval last October. Rivfloza was
okayed for children nine years and older to treat a rare genetic condition that affects the kidneys,
known as primary hyperoxaluria type 1 (PH1).View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Ionis tees up NDAs for rare
disease treatments after two late-stage trial winsThe industry is looking for cures to a wide spectrum of diseases like cancer (such as melanoma, pancreatic, liver, glioblastoma, breast and ovarian cancer), cystic fibrosis, Alzheimer’s disease, Parkinson’s disease, hepatitis B, asthma, Rett syndrome, non-alcoholic steatohepatitis, and IgA nephropathy through its research on ONTs.In January, Ionis announced late-stage results wherein its
RNA-targeted hereditary angioedema (HAE) candidate, donidalorsen, significantly
reduced the rate of HAE attacks in patients
treated every four weeks and patients treated every eight weeks. The
California-based biotech is readying a new drug application (NDA) to submit to the FDA. HAE is a rare and life-threatening genetic disease that causes unpredictable and frequent severe swelling of the skin, gastrointestinal (GI) tract, upper respiratory system, face and throat. This year, Ionis’ olezarsen was granted fast track designation by
the FDA for the rare genetic disease familial chylomicronemia syndrome (FCS).
Last September, olezarsen had met its primary endpoint of reducing abnormally high
levels of triglycerides in a late-stage trial in patients with the metabolic disorder. Currently, there are no
FDA-approved treatments for this condition. If okayed, olezarsen is likely to
bring in US $849 million in sales for Ionis by 2032.In March, FDA’s Oncologic Drugs Advisory Committee (ODAC) voted 12 to two in favor of the clinical
benefit/risk profile of imetelstat for the treatment of transfusion-dependent (TD) anemia in certain adult patients with myelodysplastic syndromes. The agency assigned a Prescription Drug User Fee Act (PDUFA) target action date of June 16, 2024, for Geron’s NDA for imetelstat. It is among the most anticipated drug launches this year.View Our Interactive Dashboard on Oligonucleotide Therapies (Free Excel Available) Our viewDeveloping ONTs is a field fraught with challenges, such as toxicity and drug delivery. There are safety concerns as well as concerns around delivering the therapy. However, technological breakthroughs and collaborations between pharma firms and contract research organizations that focus on drug delivery are continuously working towards addressing these challenges. All in all, we foresee exciting times ahead for ONTs.
Impressions: 2746
The year 2023 has
seen considerable job cuts by biopharmaceutical companies. While layoffs have
become commonplace since 2022, the exercise touched new heights this year. The reasons behind these job cuts have ranged from restructuring, drop in Covid sales, fall in quarterly revenues to shift in strategy and mergers and acquisitions.In 2022, over 100
biopharma companies had announced workforce reductions. That number has doubled in 2023. And the year has not ended as yet.In Q1 2023, the
number of companies that had announced layoffs surpassed 50 and included
companies like Biogen and Thermo Fisher. Between mid-April and November, this
figure rose even further, with approximately 140 pharmaceutical companies
disclosing workforce reductions.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Biogen, Amgen, layoff staff post mergers;
low Q2 sales trigger job cuts at BMSIn
our Q1 2023 update, we had mentioned that both Novartis and Biogen plan to cut jobs worldwide
to save US$ 1 billion in costs. Starting November, Novartis will retrench over 100 employees at its US headquarters in East Hanover (New Jersey). Similarly, Biogen
announced plans to layoff around 113 employees from Reata Pharmaceuticals’ Plano, Texas site in the US. Biogen had acquired Reata for US$ 7.3 billion in July this year, and the job cuts were announced promptly after the acquisition.Another company
that announced post-merger retrenchments is Amgen, which had acquired Horizon Therapeutics in December 2022 for US$ 27.8 billion. Amgen has been aggressively cutting
costs. Earlier this year, it had laid off 750 employees due to intensifying pressure on drug
prices and inflation. And now, it is issuing pink slips to 350 former Horizon employees.The going has
also been tough for Bristol-Myers Squibb (BMS) — it reported low Q2 revenues and had to cut its full-year forecasts
as two of its top drugs, blood cancer treatment Revlimid and blood thinner Eliquis, saw a drop in sales due to competition
from generics. As a result, BMS laid off 48 employees in New Jersey in May, and plans to do away with another 100-odd employees soon.Nearly a year
after GSK spun off its consumer healthcare
business to create Haleon, there are reports that hundreds in the
United Kingdom and potentially thousands working globally for Haleon are at the risk of losing their jobs.
The restructuring is likely to save GBP 300 million (US$ 393 million) over the next three years.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Pfizer, Thermo Fisher, Novavax announce
layoffs due to declining Covid salesIn October, Pfizer had significantly lowered its full-year revenue forecast for 2023 due to reduced demand for its Covid products. The drug behemoth has also announced cost-cutting measures such as layoffs and expense cuts that will help save US$ 3.5 billion.During the same
month, Pfizer announced plans to retrench 791 employees at its Gladstone site in the US. A month
later, the company announced 800 job cuts, by reducing staff at its Kent (the UK), Michigan (the US), and Newbridge, Kildare (Ireland) sites.Thermo Fisher Scientific has been downsizing its workforce at
different locations since last year. The company manufactures Covid testing
kits. In our Q1 2023 update, we had mentioned that the company laid off around 500 employees across various locations in California between January 2022 and mid-April 2023.In a fresh wave
of job cuts announced in November, Thermo Fisher will layoff 97 employees in January 2024. The company plans to
close its facility in Alabama. Earlier in August, the company fired 205 staffers across two separate sites in Alachua, Florida, due to the relocation of development, manufacturing, and production activities from this site to their new Plainville, Massachusetts site.In November,
Covid vaccine maker Novavax announced second round of cost cuts for 2023 in order to reduce spending by US$ 300 million. In May, Novavax had reduced its
headcount by 25 percent to align its spending with the diminishing size of the Covid opportunity.Similarly, in
June, contract manufacturer Catalent said it plans to retrench 150 employees at its Bloomington, Indiana plant.
Catalent has been working closely with drugmakers to manufacture Covid vaccines
and therapies, and the job cuts are part of its post-pandemic restructuring
exercise.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Shifts in strategy, restructuring trigger layoffs at Takeda, PTC Therapeutics, ApellisIn May, Takeda had announced a shift in its R&D strategy, leading to 186 layoffs in Massachusetts and an additional 27 in San Diego. This announcement follows Takeda’s decision to discontinue discovery and pre-clinical efforts in AAV (adeno-associated virus) gene therapy and rare hematology.In a similar move
announced in September, rare disease drugmaker PTC Therapeutics announced retrenchment of 25 percent of its workforce as part of a broader restructuring initiated in May, involving discontinuation of several early-stage gene therapy development programs to focus on high-potential R&D programs. PTC plans to complete the process by January 15. In August, Apellis announced its plan to layoff
approximately 225 employees and divest two preclinical assets to
achieve US$ 300 million in savings as part of a significant corporate
restructuring program. This move aims to drive the growth of Syfovre, an
injectable form of pegcetacoplan that received FDA approval for geographic atrophy (GA) in February.During the same
month, Sage Therapeutics, a company working on novel therapies
for brain health disorders, announced 188 job cuts. These were part of a restructuring plan announced soon after FDA rejected its
drug Zurzuvae (developed along with Biogen) for major depressive disorder (MDD). The agency has approved the pill for postpartum depression, which is a much smaller market as compared to MDD.In September, 2seventy bio (a company spun out of bluebird bio) had announced plans to layoff about 40 percent of its workforce (i.e. axe 176 jobs) in order to lower costs and focus on the biotech firm’s cancer cell therapy — Abecma.In August, Emergent BioSolutions decided to cut 400 jobs and scale back operations at some of its
facilities, in an effort to move away from its CDMO business and pivot its focus on core products, such as
nasal spray Narcan and anthrax vaccines.View Our Interactive Dashboard on Biopharma Layoffs in 2023 (Free Excel Available)Our viewThe
biopharmaceutical industry is still grappling with the disruptions caused by
Covid. Some of the layoffs reflect a correction, as demand moves back to
pre-pandemic days. The others are either a fallout of disappointing quarterly
results, or the outcome of normal business restructuring and strategy shifts.While this
article was going to print, there was news that Kite Pharma, a subsidiary of Gilead focusing on cell therapies, is letting
go off 7 percent of its staff, or around 300 employees in order to improve
operational efficiency. Similarly, another genetic medicines company Generation Bio is firing 68 employees. Both these updates follow news that FDA is investigating the “serious risk” of cancer patients developing secondary blood cancer after undergoing chimeric antigen receptor T-cell (CAR-T) therapies. Incidentally, several startups focusing on cell and gene therapies (such as
Summation Bio, Oncurus Inc, Intergalactic Therapeutics), have had to shut operations due to lack of finance.As we bid
farewell to 2023, there are larger concerns of a slowdown in the global economy. For now, it seems
like 2024 may not be very different from 2023.
Impressions: 6639