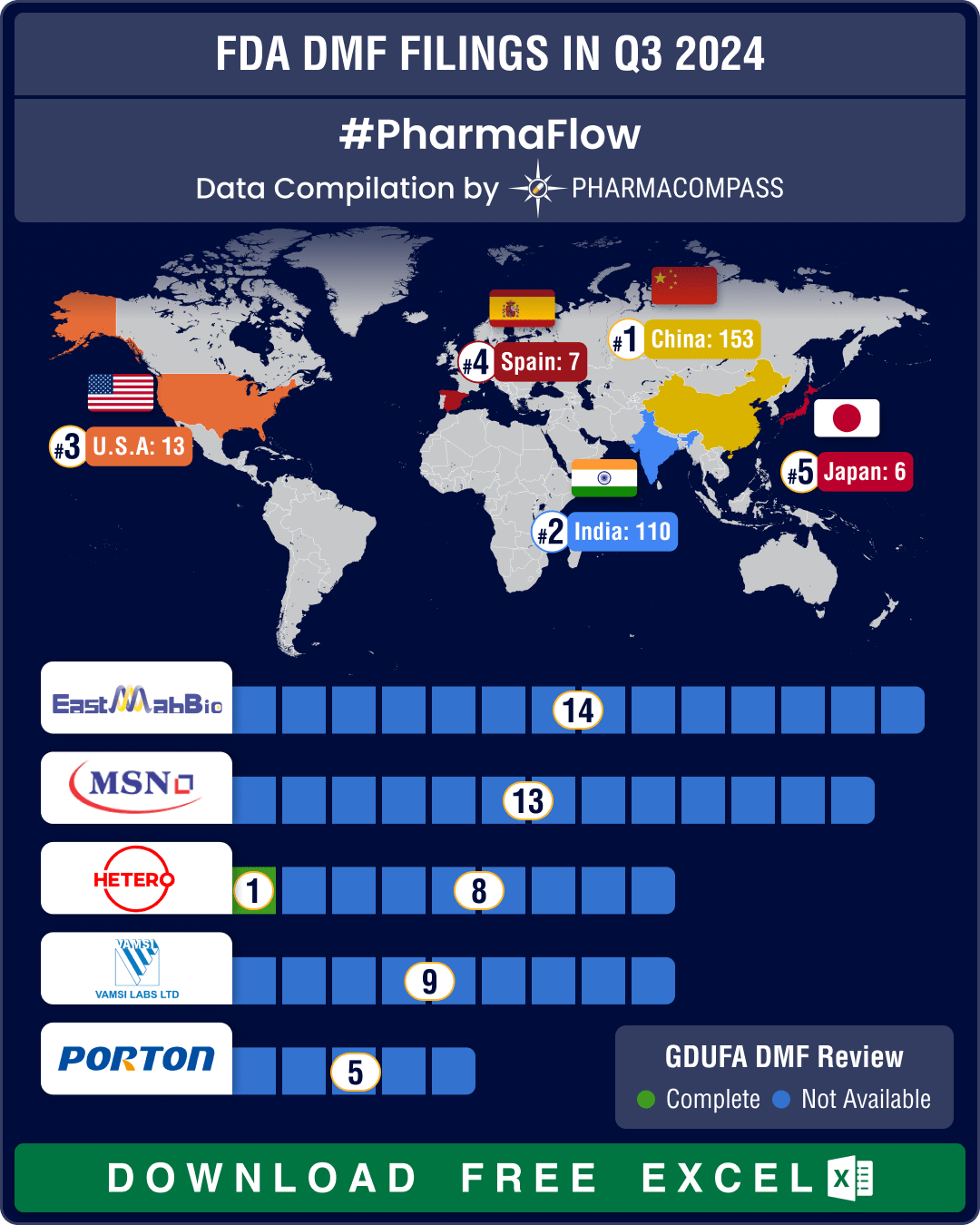
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica
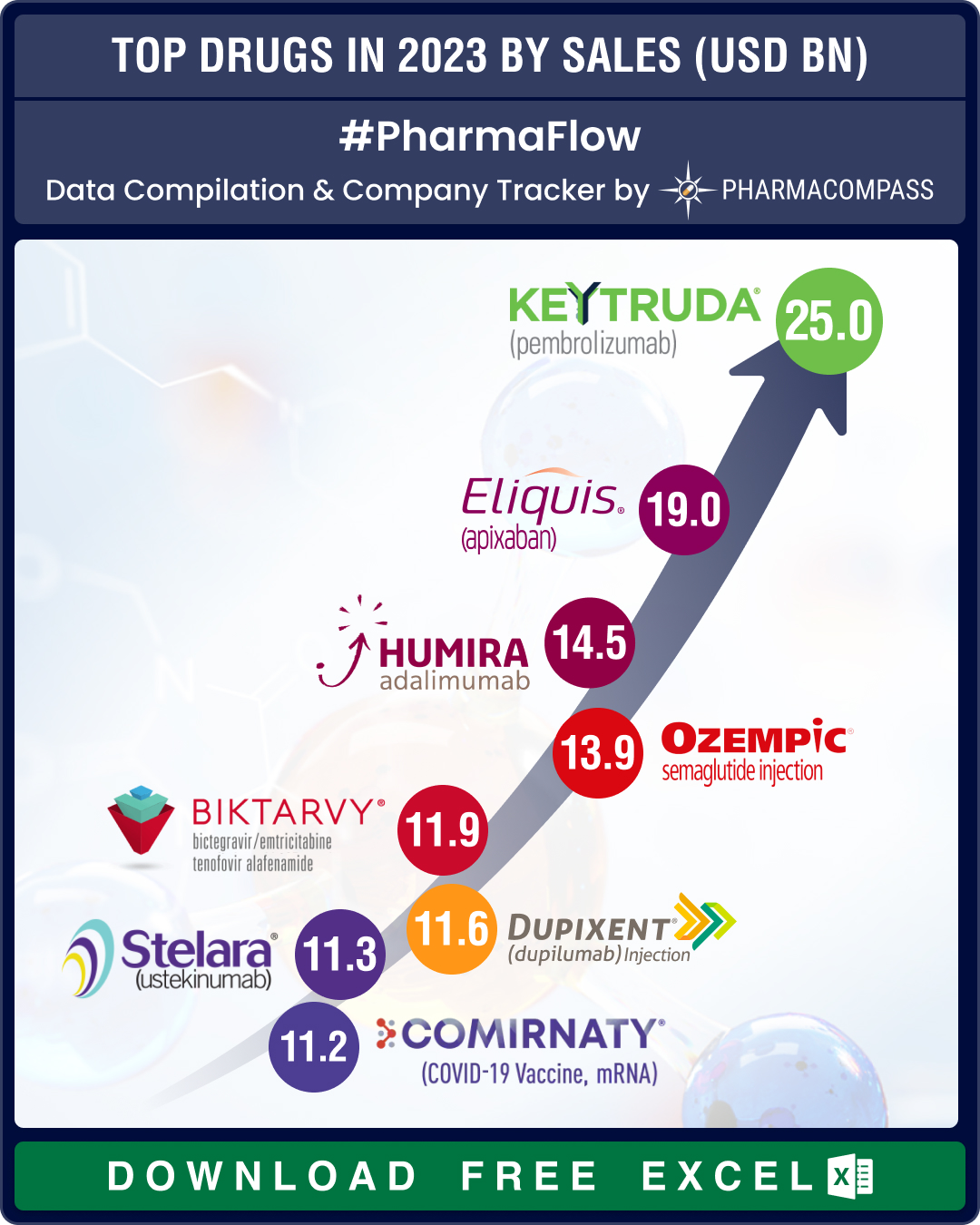
Top Pharma Companies & Drugs in 2023: Merck’s Keytruda emerges as top-selling drug; Novo, Lilly sales skyrocket
The pharma industry clearly recalibrated itself in 2023, turning its focus away from Covid and onto


 Market Place
Market Place Sourcing Support
Sourcing Support