Top drugs and pharmaceutical companies of 2019 by revenues
Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-
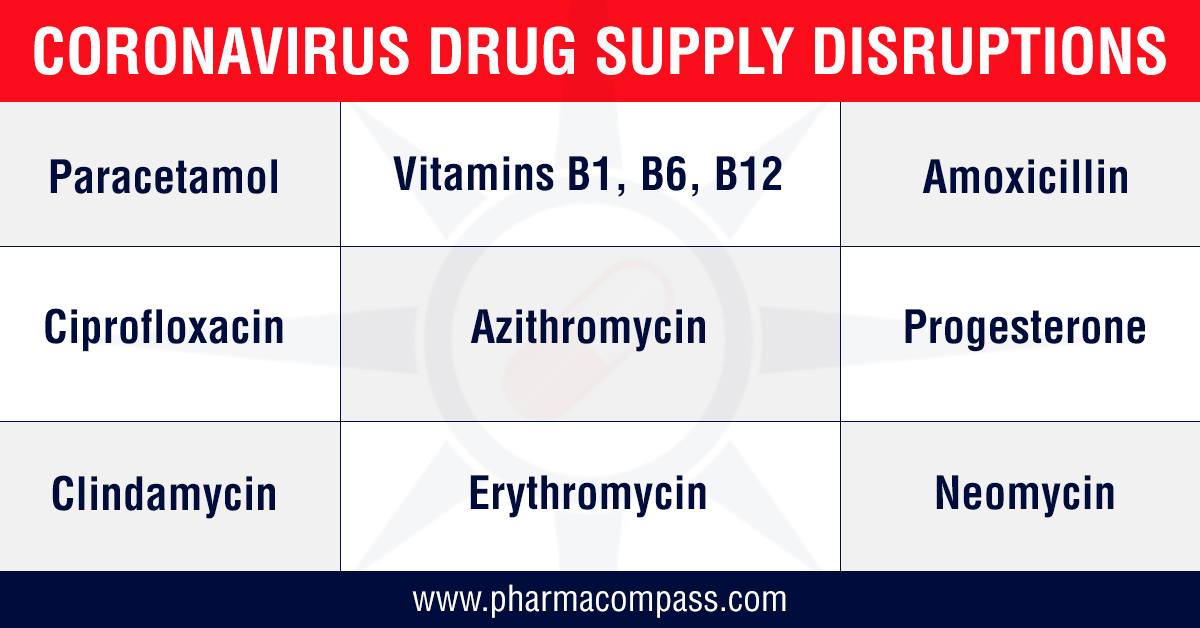
COVID-19: India restricts drug exports amid rising prices of essential bulk drugs; FDA announces first drug shortage
Now that it has been
established that the novel coronavirus is going to globally impact the drug
s
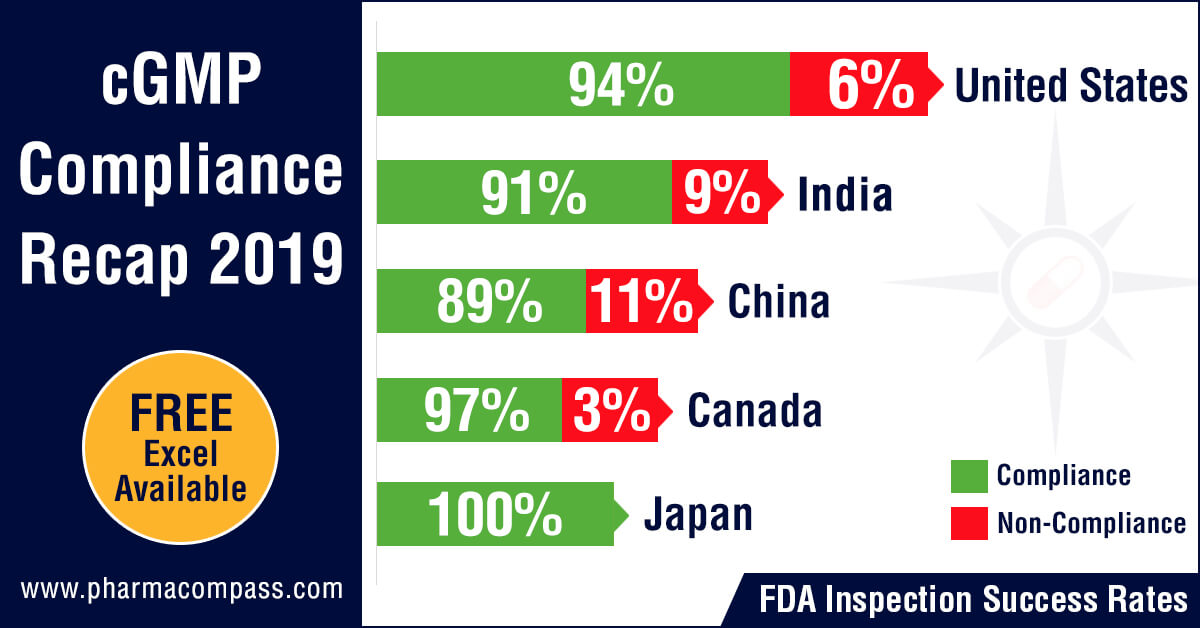
cGMP Non-Compliance Recap 2019
In 2019, concerns over quality of medicines continued to dominate news headlines. The ‘sartan&
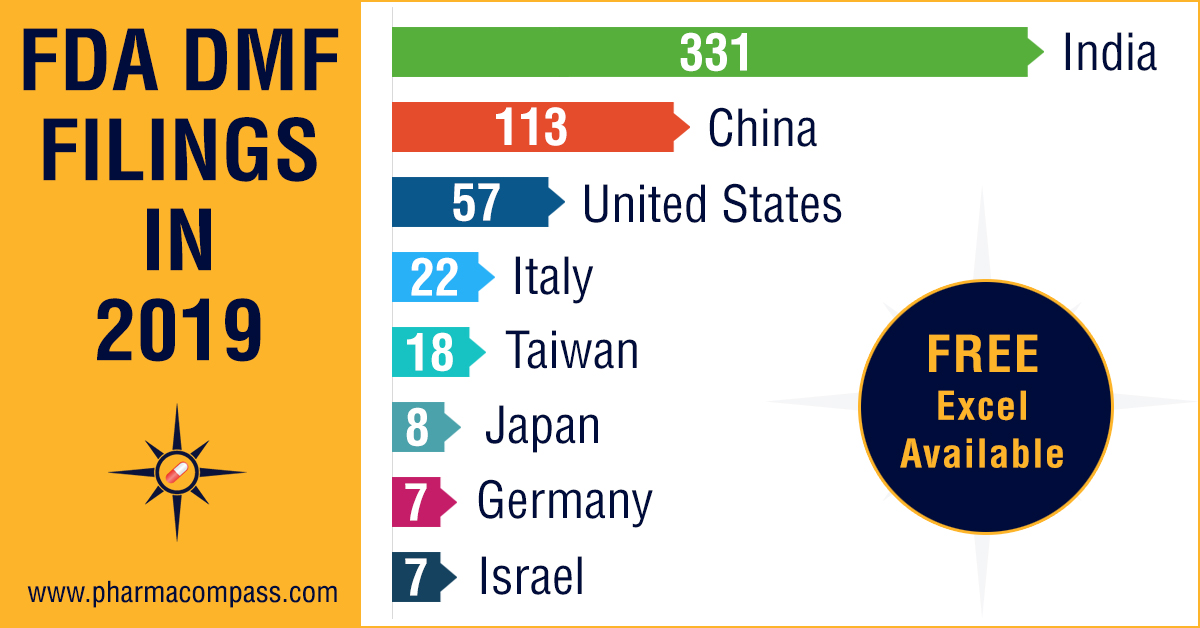
DMF submissions in 2019: India maintains bulk drug supply supremacy to US
At PharmaCompass, we highlighted
the significance of India in the global active pharmaceutical ingr
FDA bans import of all APIs from Zhejiang Huahai; EU issues non-compliance certificate
In case you thought the US Food and Drug Administration (FDA) and EU’s actions against China&r
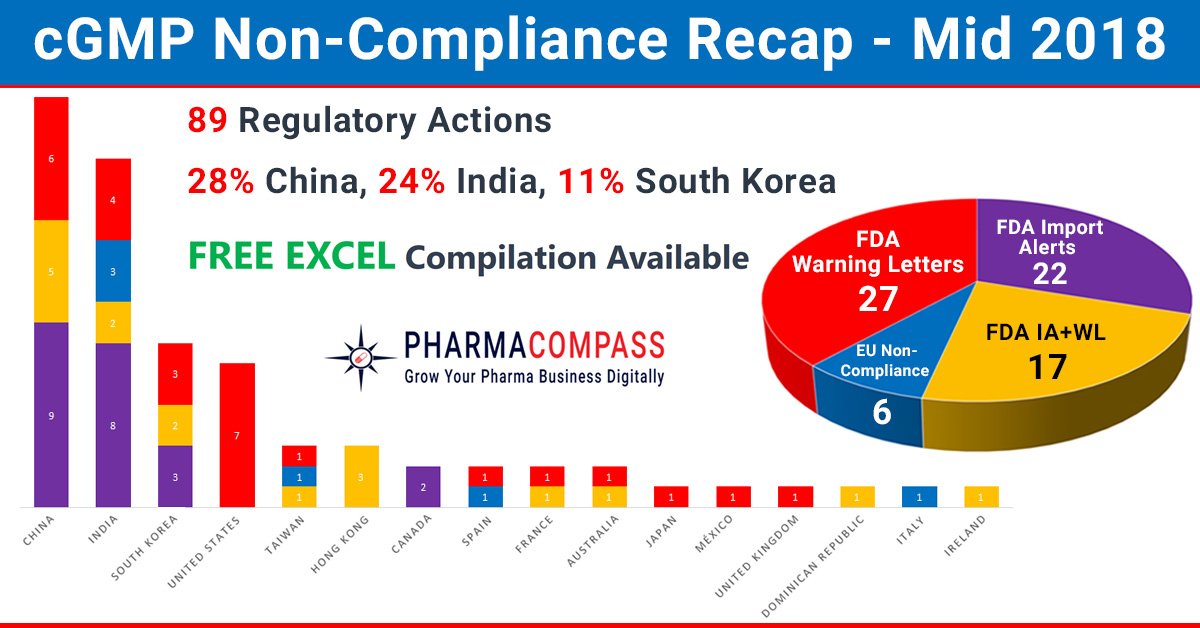
Mid 2018 – Recap of Warning Letters, Import Alerts and Non-Compliances
In our mid-2018 compliance review, we look at inspection challenges
faced by companies across the w
Trumpcare receives a blow; EMA to suspend 300 drugs due to clinical data integrity violations in India
This week, Phispers takes you to the US, where President Trump’s efforts to repeal and replace
Will data integrity concerns on clinical trials done at GVK Biosciences go beyond Europe?
Over
700 commonly used generic medicines were
recommended for suspension by the European Medic
Dr. Reddy’s largest API facility maybe the next to get banned from exporting to the United States
Dr. Reddy’s largest active pharmaceutical ingredient
(API) plant in Srikakulam (Andhra Pradesh



 Market Place
Market Place Post Enquiry
Post Enquiry