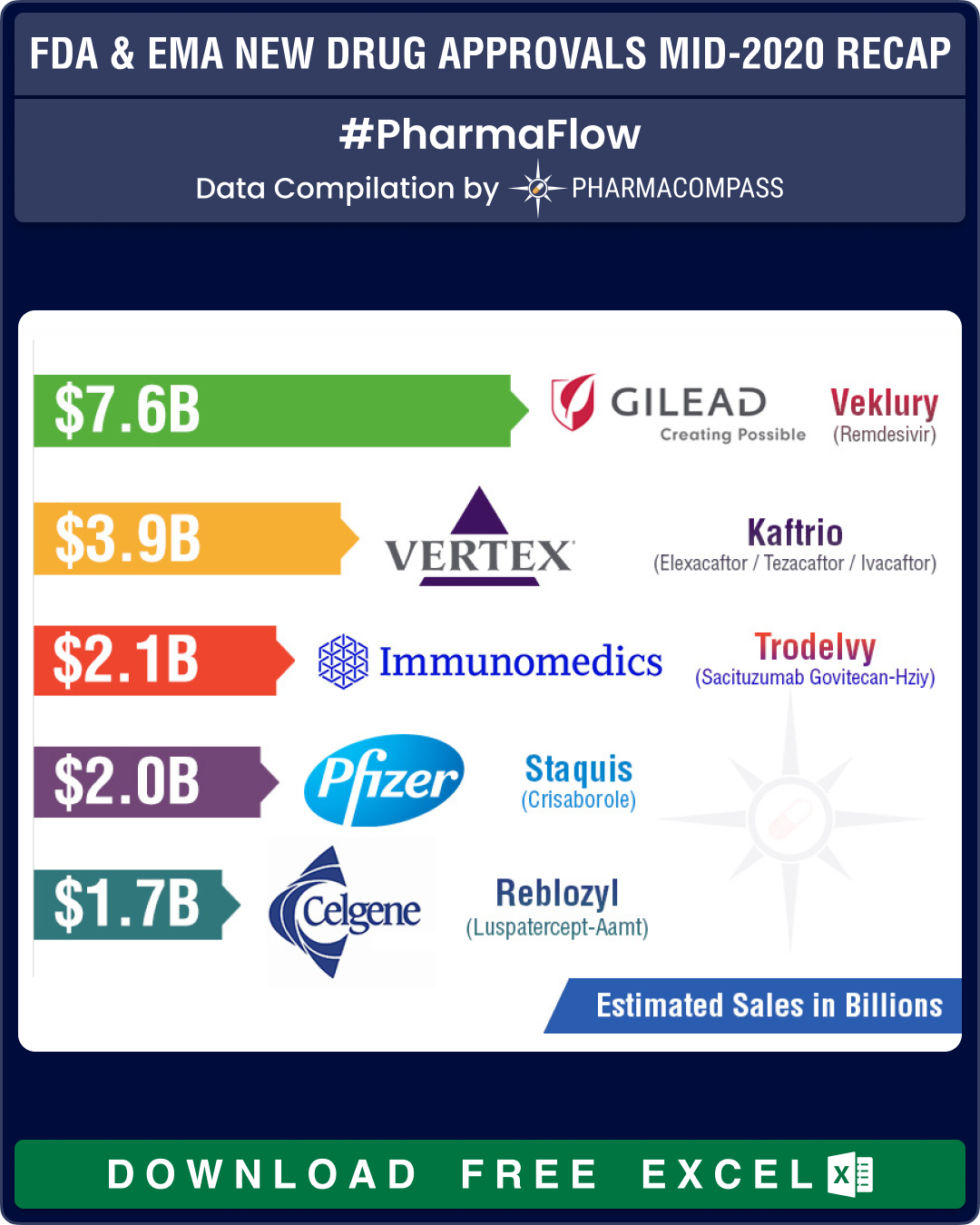
New Drug Approvals by FDA & EMA (Mid-2020 Recap)
In case you thought Covid-19 had slowed down US Food and Drug Administration’s New Drug Approv
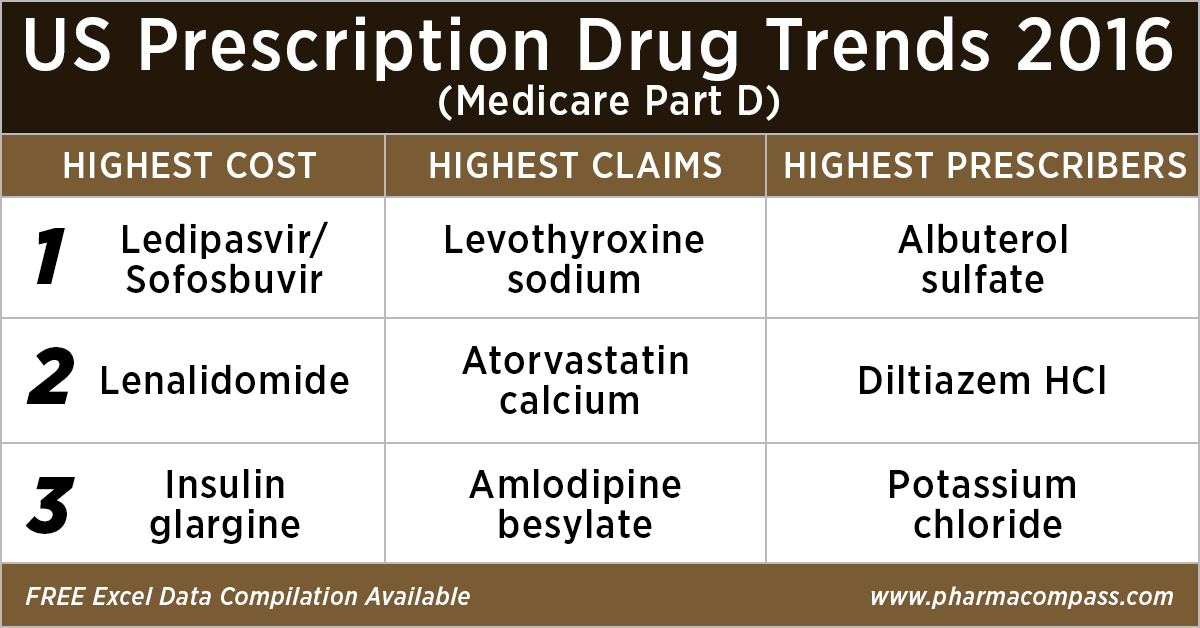
Analyzing over US$ 90 billion of Medicare Prescription Drug (Part D) Spending in 2016
This week, PharmaCompass
reviews the recently released data on prescription drugs paid for under th
Pfizer exits China venture with Hisun; FDA mulls more steps to increase generic drug competition
This week in Phispers, we look at the measures US FDA announced last week to increase competition in
Trumpcare receives a blow; EMA to suspend 300 drugs due to clinical data integrity violations in India
This week, Phispers takes you to the US, where President Trump’s efforts to repeal and replace


 Market Place
Market Place Sourcing Support
Sourcing Support