By PharmaCompass
2025-03-06
Impressions: 856 (Article) || 86 (Video)
AbbVie has forayed into the obesity treatment market with a US$ 2.23 billion licensing deal with Denmark’s Gubra for its novel long-acting amylin drug, GUB014295. A study published in The Lancet says 50 percent of the world’s population may be overweight or obese by 2050, slamming soaring global obesity rates as a “monumental societal failure”.
Roche’s Genentech broke a 30‐year dry spell in stroke care by securing a label expansion from the US Food and Drug Administration for its cardiac med TNKase. The five‐second IV TNKase injection is now approved for treating acute ischemic stroke. In deals, Eli Lilly has inked a US$ 1.2 billion deal with Magnet Biomedicine to harness its innovative molecular glue technology for oncology applications.
In the US, the Department of Government Efficiency (DOGE) is reportedly terminating the leases of 30 FDA sites across 23 states. And Health Secretary Robert F. Kennedy (RFK) Jr. has proposed scrapping public comments for decisions made by the US Department of Health and Human Services (HHS).
In trials, AstraZeneca and Amgen’s investigational biologic Tezspire has delivered impressive phase 3 results in patients with chronic rhinosinusitis with nasal polyps. Meanwhile, Biohaven’s late-stage trial for its bipolar candidate BHV-7000 failed to meet its primary endpoint.
And Glenmark Pharmaceuticals issued a recall of nearly 1.5 million bottles of its generic ADHD drug atomoxetine after detecting levels of a probable carcinogen, N-Nitroso atomoxetine, that exceed FDA limits.
AbbVie enters obesity race with US$ 2.23 bn deal with Denmark’s Gubra for long-acting amylin drug
AbbVie has forayed into obesity treatment by signing a licensing agreement with Danish biotech firm Gubra for its innovative amylin-targeting drug, GUB014295. AbbVie will make an upfront payment of US$ 350 million and could pay an additional US$ 1.875 billion in milestone payments.
The phase 1 GUB014295 represents a novel approach in the obesity arena, as it targets the amylin hormone — a mechanism distinct from the popular GLP-1-based therapies currently dominating the market. Analysts have called the amylin pathway the “hottest new mechanism for obesity” with potential for greater reductions in fat instead of lean muscle.
Study says over 50% of adults may become overweight by 2050: A new study published in The Lancet says the rates of overweight and obesity have more than doubled over the past three decades due to a “monumental societal failure”. As of 2021, 2.1 billion adults (up from 731 million in 1990) and 493 million young people (up from 198 million in 1990) were classified as overweight or obese. Without urgent and comprehensive interventions, the study projects that by 2050 more than 3.8 billion adults — i.e. over half of the world’s adult population — and 746 million children and adolescents could be affected by it.
DOGE to terminate leases of 30 FDA sites across 23 US states
The US Department of Government Efficiency under billionaire Elon Musk is reportedly terminating the leases of 30 FDA sites across 23 states as part of cost-cutting efforts. It wasn’t immediately clear when any of these terminations are set to take effect. Initially, a 52,000 square-foot St. Louis, Missouri-based drug testing facility, known as the most important FDA drug testing lab, was included among the closures. However, reports now suggest that facility will stay open.
RFK Jr proposes scrapping public comment on major US health policies: RFK Jr. has proposed ending the public comment period for decisions made by the HHS. Ironically, RFK Jr has pledged “radical transparency”. The public comment policy has been in place since 1971, ensuring that diverse perspectives are considered in policy making.
FDA expands label of Genentech’s TNKase, ends 30-year drought in stroke treatment
Roche subsidiary Genentech has won FDA approval for its stroke treatment TNKase (tenecteplase). Originally approved for treating acute myocardial infarction (heart attack), TNKase has become the first new medicine for treating acute ischemic stroke (AIS) in adults in nearly 30 years. The key advantage of TNKase lies in its streamlined administration — it is delivered as a single five-second shot in the arm. This rapid and simple method can be critical in acute stroke management.
Lilly inks US$ 1.2 bn deal with Magnet in hot molecular glue space
Eli Lilly has promised Boston-based Magnet Biomedicine over US$ 1.2 billion in addition to a US$ 40 million in upfront payment to use its TrueGlue discovery technology to discover, develop, and commercialize molecular glue therapeutics in oncology. This rapidly emerging field has been attracting significant investment. TrueGlue harnesses small molecules that act like a “molecular glue” by binding proteins together in new ways to correct faulty cell processes.
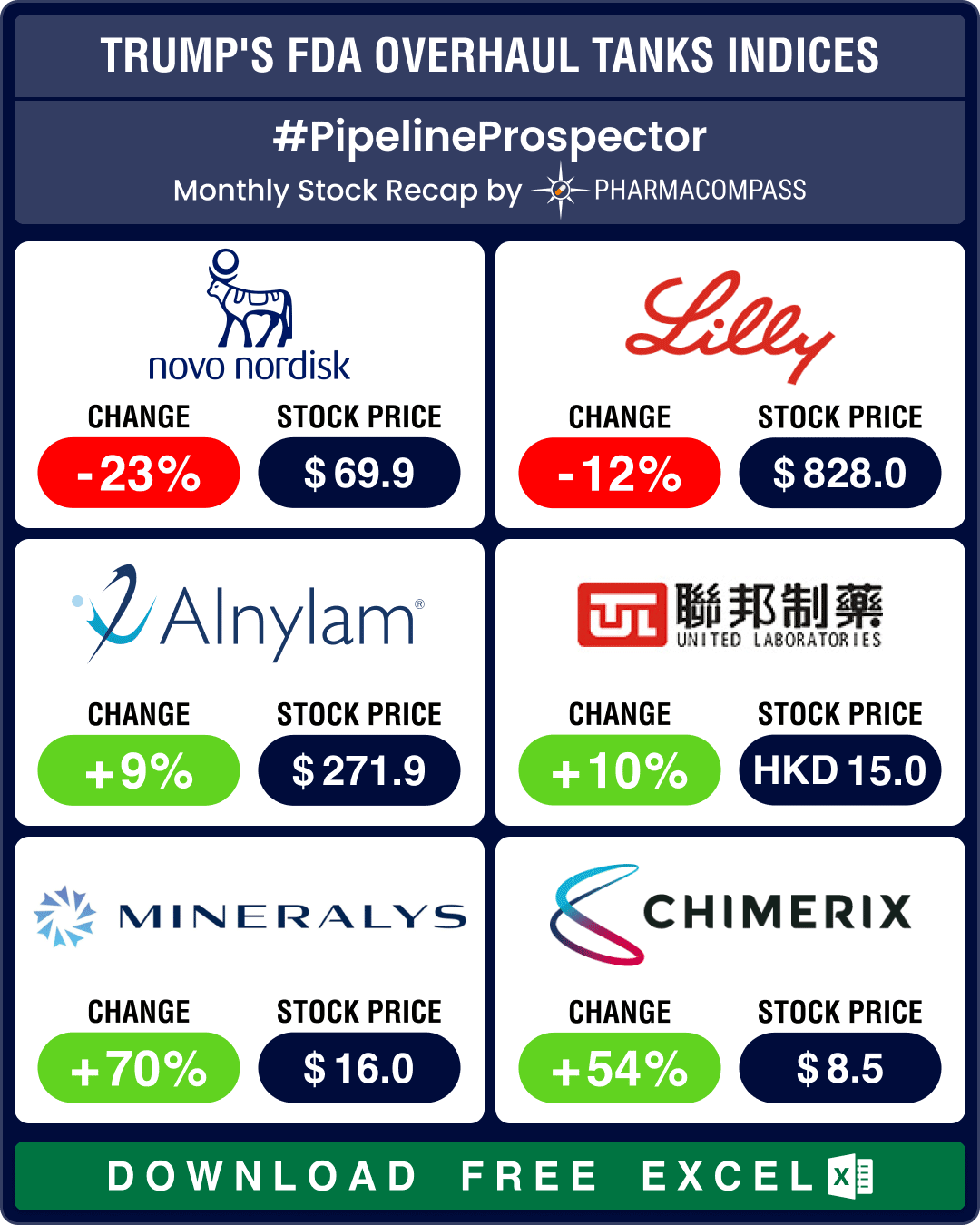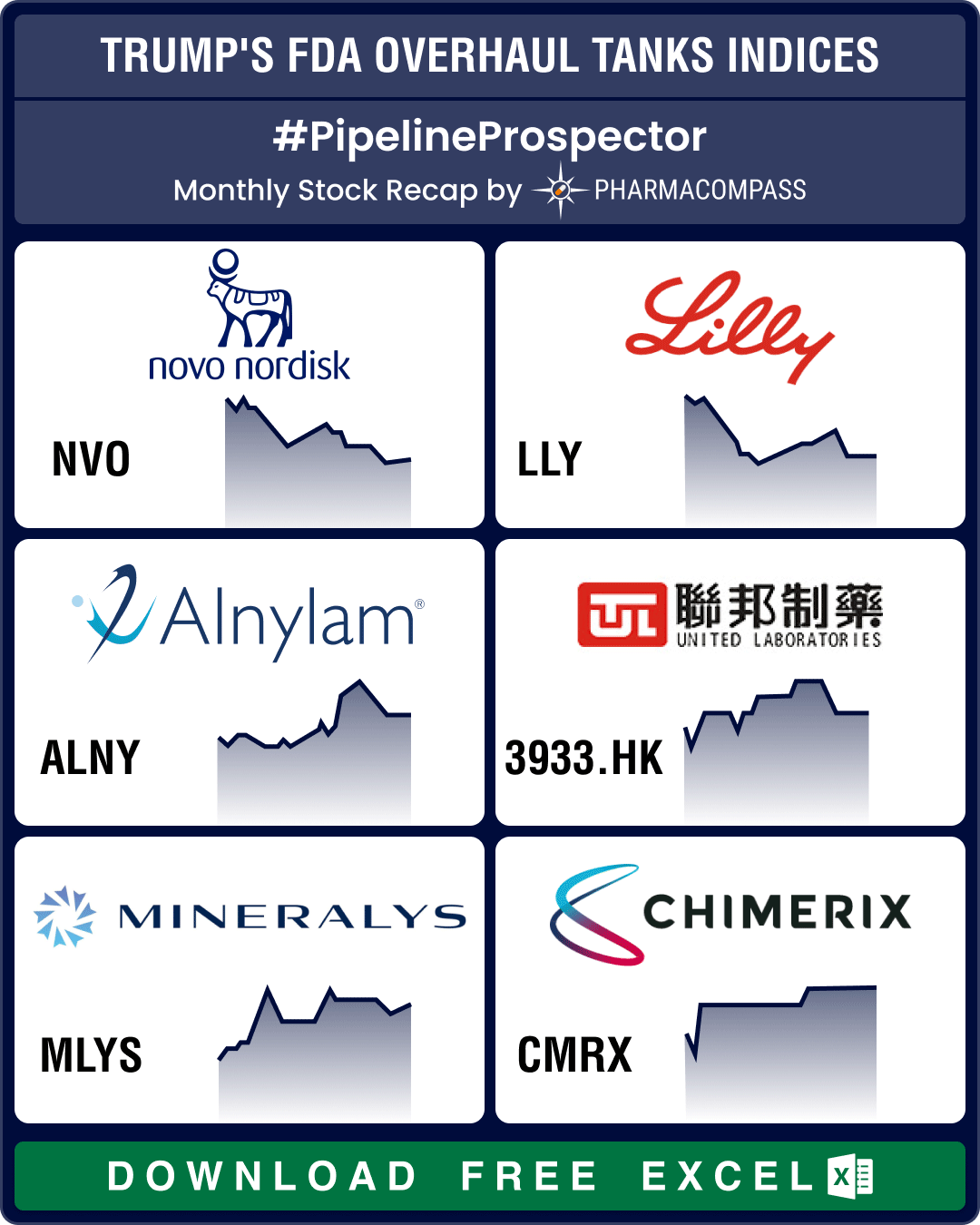
Jazz to buy Chimerix for US$ 935 mn: Jazz Pharmaceuticals is acquiring Chimerix for approximately US$ 935 million. Chimerix’s lead candidate, dordaviprone, is under FDA review for the treatment of a rare brain tumor, known as H3 K27M-mutant diffuse glioma.
Astra, Amgen see impressive late-stage results for Tezspire in chronic sinusitis
In a breakthrough for chronic sinusitis treatment, Amgen and AstraZeneca announced that their investigational biologic Tezspire (tezepelumab-ekko) has delivered impressive results in a late-stage trial for patients with chronic rhinosinusitis with nasal polyps (CRSwNP). Notably, Tezspire nearly eliminated the need for subsequent nasal polyp surgery, achieving a 98 percent reduction, while also cutting systemic corticosteroid use by 88 percent. Amgen and AstraZeneca aim to file for regulatory expansion in CRSwNP in the first half of 2025.
Biohaven’s bipolar candidate fails to treat mania in late-stage trial
Biohaven’s pivotal late-stage trial for its bipolar candidate BHV-7000 did not meet its primary endpoint, delivering a significant setback for the company’s efforts to treat mania symptoms. Biohaven emphasized that the drug was safe and well tolerated, and the company has decided not to pursue further bipolar indications for BHV-7000. Instead, Biohaven will continue evaluating the drug in other therapeutic areas, such as major depressive disorder and epilepsy.
Glenmark recalls nearly 1.5 million bottles of generic ADHD drug
Glenmark Pharmaceuticals has issued a recall of nearly 1.48 million bottles of its generic ADHD
medication, atomoxetine. The recall was initiated
due to the detection of a possible carcinogen, N-Nitroso atomoxetine, which
exceeds the limits recommended by the FDA.
New Keytruda version may face patent challenge: A new injected version of Merck’s blockbuster cancer drug Keytruda (pembrolizumab), currently in clinical testing, could face a patent challenge from biotech Halozyme Therapeutics. Halozyme says the new version infringes on its patents.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Phisper Infographic by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”