By PharmaCompass
2024-09-12
Impressions: 1,010 (Article) || 15 (Video)
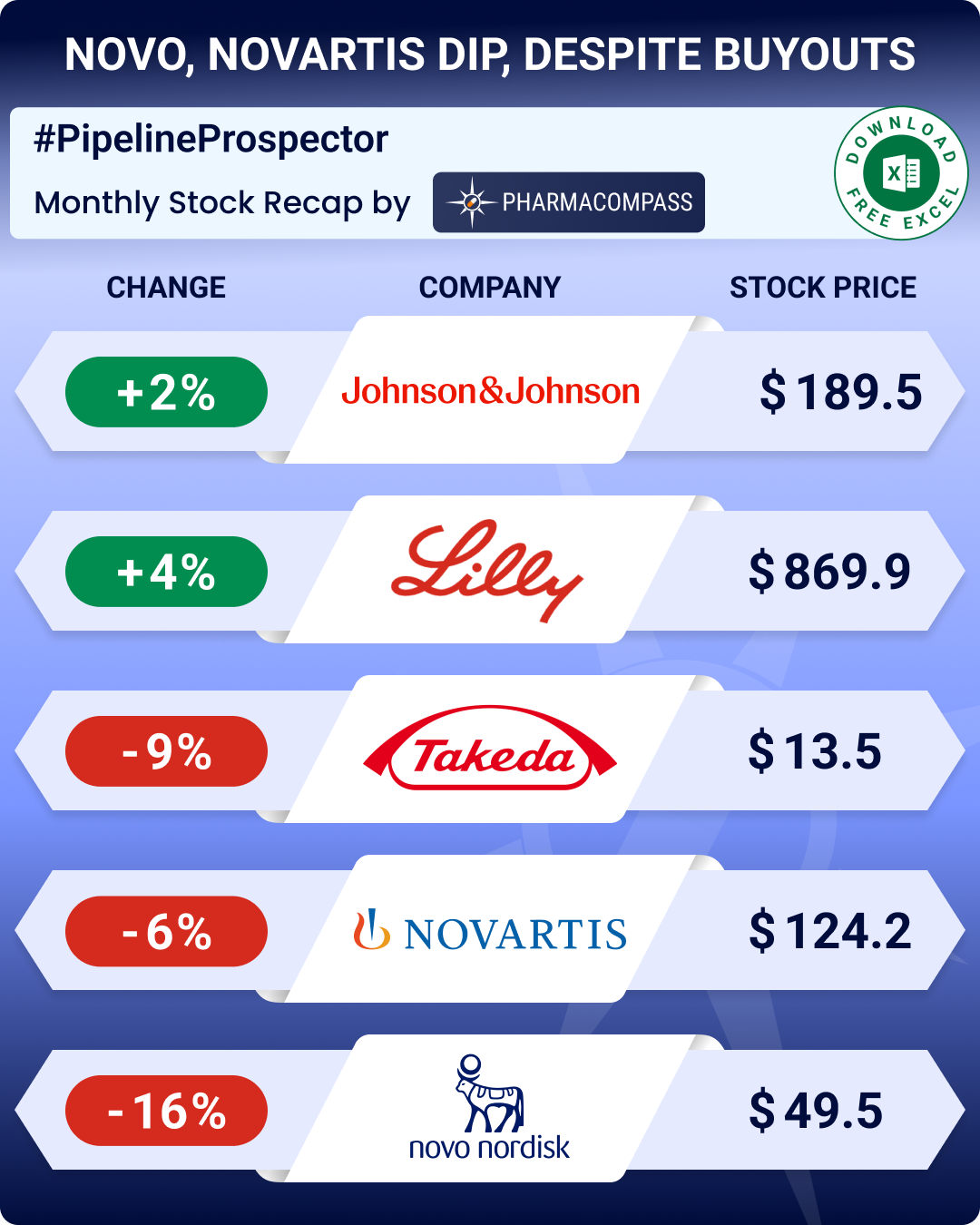
GSK scored a significant late-stage win with its asthma drug Nucala in treating chronic obstructive pulmonary disease (COPD). It also posted positive results from a late-stage trial on its long-acting asthma medication depemokimab. However, GSK’s experimental herpes simplex virus vaccine failed to meet its main goal. The drugmaker has decided not to proceed with a late-stage trial for the vaccine.
Novo Nordisk made strides in addressing childhood obesity with its drug Saxenda, which has been in use for many years and has a well-established safety record.
AstraZeneca and Daiichi Sankyo faced disappointment with their antibody-drug-conjugate (ADC) in lung cancer trials. However, Akeso and Summit Therapeutics’ ivonescimab beat Merck’s blockbuster Keytruda in a late-stage trial.
In policy news, the US House of Representatives passed the Biosecure Act, which aims to restrict business with several Chinese biotech companies citing national security concerns.
Meanwhile, FDA has issued a warning letter to India’s Zydus Lifesciences, citing significant violations of current good manufacturing practice (CGMP) regulations at its Gujarat (India) plant. And Lykos Therapeutics’ CEO has stepped down in the latest hit to the company, after its MDMA drug setback.
GSK’s asthma drug Nucala scores late-stage win as COPD race heats up
GSK’s asthma drug Nucala (mepolizumab) has scored a vital win in a late-stage study in treating COPD patients. The British drugmaker said the monoclonal antibody met its primary endpoints, wherein it significantly and meaningfully reduced the annualized rate of moderate/severe exacerbations. COPD is the world’s third leading cause of death. GSK’s results come after FDA approved Verona’s Ohtuvayre (ensifentrine) as the first inhaled product with a novel mechanism of action available for the maintenance treatment of COPD in more than 20 years.
Meanwhile, Sanofi and Regeneron’s blockbuster Dupixent (dupilumab) has an FDA target action date of September 27 as a COPD treatment.
Long-acting asthma drug halves attacks: Another monoclonal antibody from GSK and a new long-acting asthma drug — depemokimab — reduced asthma exacerbations by 54 percent, meeting the primary endpoint of a late-stage trial. Depemokimab also met its secondary endpoint by achieving a 72 percent reduction in exacerbations that required hospitalization or an ER visit. GSK is counting depemokimab among one of its 12 blockbuster launches and expects it to generate annual peak-sales of £ 3 billion (US$ 3.9 billion).
Ditches experimental herpes vaccine: GSK’s experimental herpes simplex virus (HSV) vaccine failed to meet its primary goal in a mid-stage trial and the drugmaker will not proceed with a late-stage trial. Currently, there are no approved vaccines for the virus that causes genital herpes.
Novo’s weight-loss drug Saxenda found to be safe, effective at reducing BMI of kids
Novo Nordisk’s older weight-loss drug Saxenda (liraglutide) has shown promising results in safely treating obesity in children aged six to 12 years. This marks a significant development in the field of pediatric obesity treatment, as currently no medications are approved for children under 12 years. The 56-week trial involved 92 children and demonstrated that those receiving daily injections of Saxenda experienced a 7.4 percent reduction in BMI compared to a placebo group. These results are particularly noteworthy given the rising rates of childhood obesity. However, experts have cautioned about the long-term effects of such treatments on children’s development.
Akeso-Summit’s lung cancer candidate scores win over Merck’s Keytruda
Akeso and Summit Therapeutics’ experimental drug ivonescimab beat the world’s best-selling drug Keytruda hands down in a late-stage NSCLC trial. Ivonescimab reduced the risk of disease progression or death by 49 percent compared to Keytruda (pembrolizumab). The drug also showed better results in terms of overall response rate and disease control rate.
FDA expands use of J&J’s Tremfya: The US drug regulator has expanded the use of Johnson & Johnson’s drug Tremfya to treat adults with chronic inflammatory bowel disease (IBD). With this approval, Tremfya is now approved for the treatment of plaque psoriasis, active psoriatic arthritis and ulcerative colitis (a chronic IBD). The expanded approval was based on data from an ongoing phase 2b/3 study.
Astra-Daiichi’s ADC flunks phase 3 lung cancer trial ahead of FDA decision
AstraZeneca said its investigational ADC failed to reach statistically significant results in a late-stage lung cancer trial, compared to the standard-of-care chemotherapy docetaxel. The trial was closely watched with analysts expecting datopotamab deruxtecan (Dato-DXd) to potentially become one of Astra’s best-selling drugs. However, Dato-DXd achieved mean overall survival of 12.9 months compared to 11.8 months for docetaxel in adult patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) treated with at least one prior line of therapy. Dato-DXd is being jointly developed by Astra and Daiichi Sankyo. FDA has set December 20 as the target action date for approving Dato-DXd as an NSCLC treatment.
Despite this setback, Astra and Daiichi unveiled a novel AI-powered biomarker that may predict which NSCLC patients are more likely to benefit from Dato-DXd treatment.
US Biosecure Act to restrict business with Chinese firms passes House
The US House of Representatives has passed the Biosecure Act, a bill aimed at restricting business with several Chinese biotech companies, including WuXi AppTec, WuXi Biologics, BGI Group, MGI, and Complete Genomics, citing national security concerns. The bill passed with a significant majority of 306 to 81 votes, demonstrating strong bipartisan support. This legislation would prohibit federal contracts with targeted firms and those doing business with them, with the stated goal of protecting Americans’ personal health, genetic information, and US pharmaceutical supply chains. The bill’s passage in the House is a crucial step, but it still needs to clear the Senate and receive President Biden’s signature to become law.
FDA issues warning letter to India’s Zydus over glass particulate contamination
FDA has issued a warning letter to India’s Zydus Lifesciences, citing significant violations of current good manufacturing practice (CGMP) regulations at its Waghodia plant in Gujarat (India). Among the key violations highlighted is Zydus’ failure to thoroughly investigate unexplained discrepancies, including cross-contamination events and glass particulate contamination in multiple drug batches. The agency chided the company over its handling of glass particulate contamination in cyanocobalamin injections, used to treat vitamin B12 deficiency. FDA also found the company’s response to a Form 483 to be inadequate.
Lykos’ CEO steps down: Lykos Therapeutics announced that CEO Amy Emerson will step down. This move follows recent turmoil at Lykos, including a 75 percent workforce reduction and founder Rick Doblin’s departure from its board. All these developments have taken place after FDA rejected Lykos’ MDMA-based PTSD treatment.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Phisper Infographic by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”