By PharmaCompass
2023-05-25
Impressions: 2,464
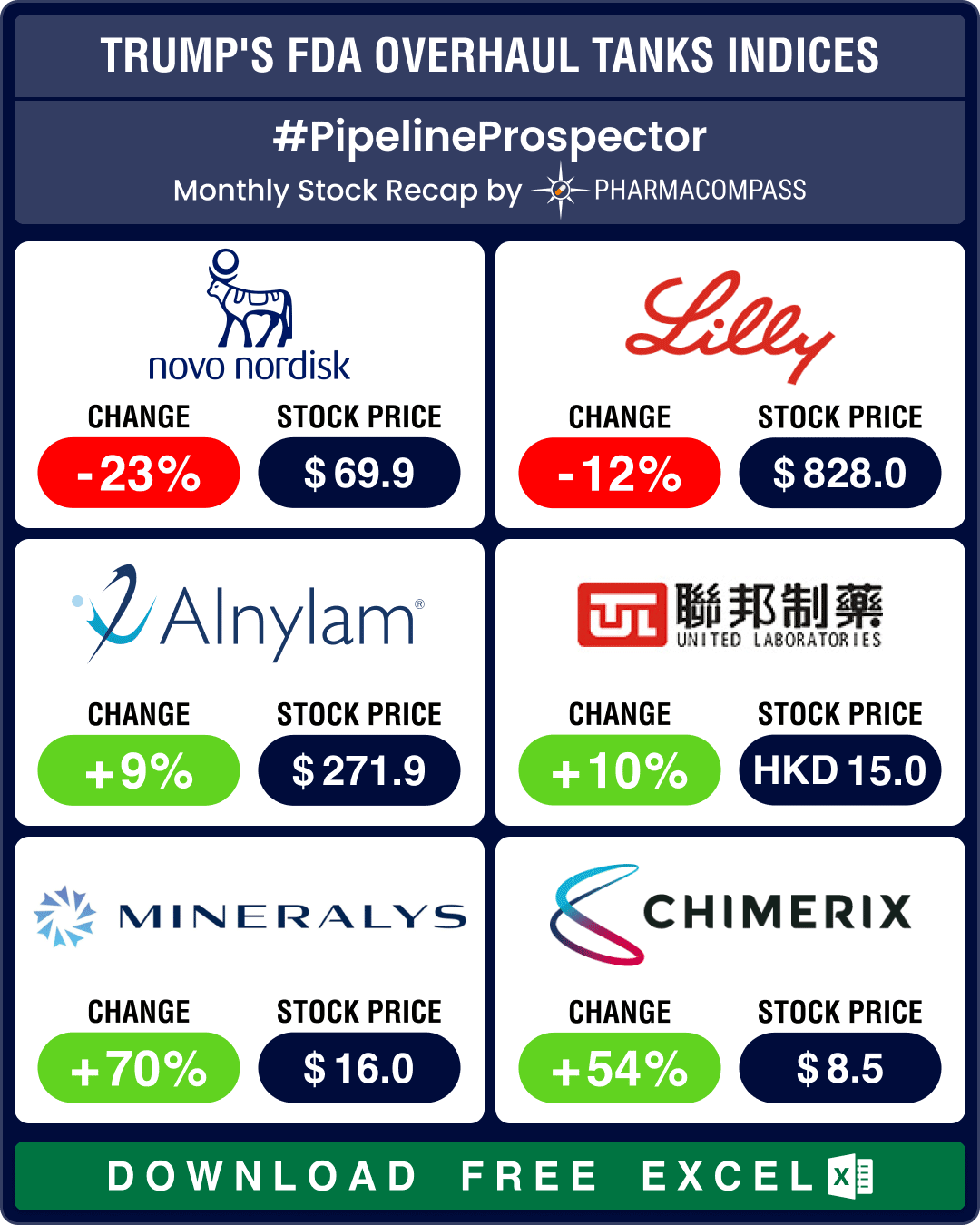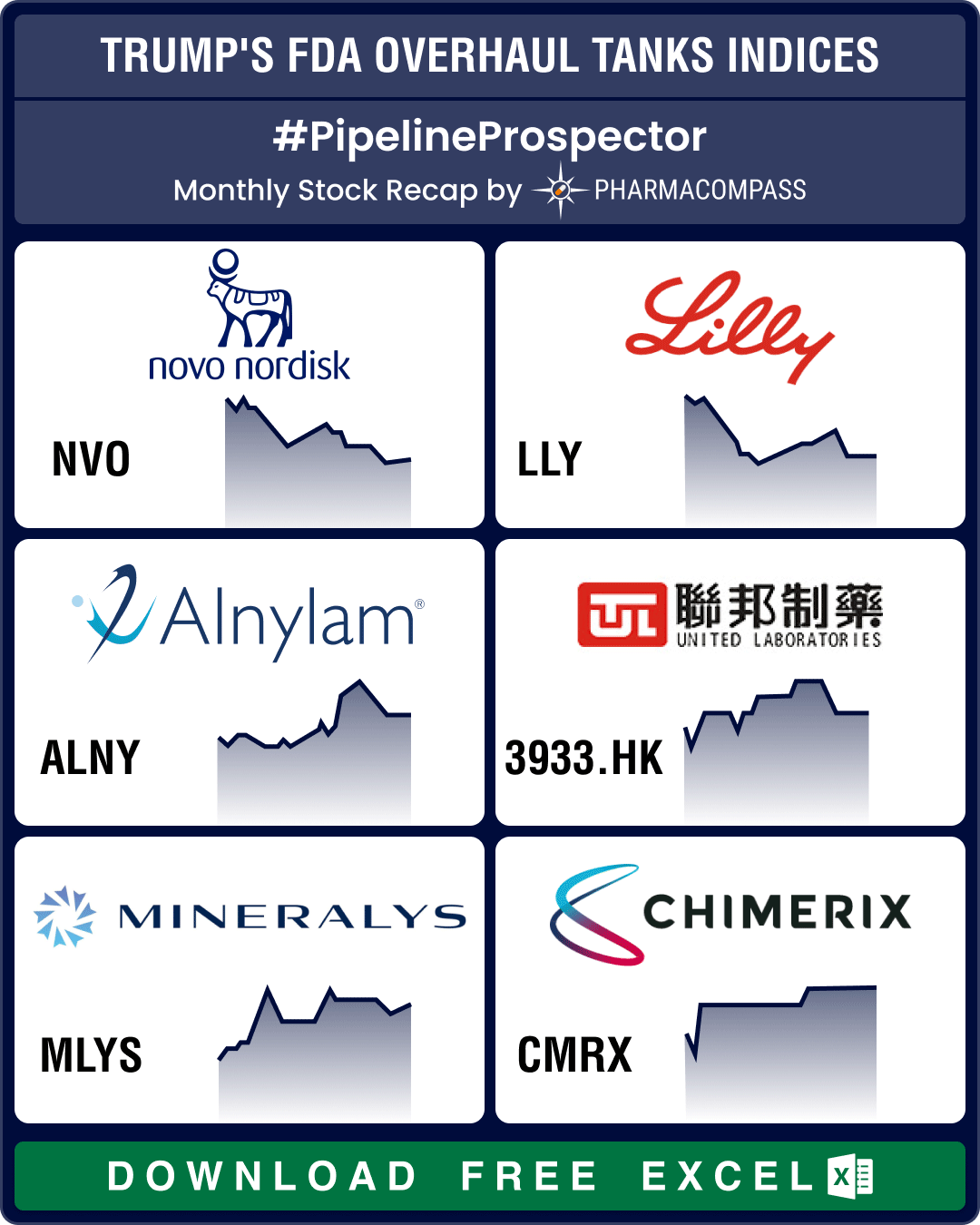
Amid soaring demand from weight watchers, Novo Nordisk has temporarily halted all television advertising campaigns for its blockbuster obesity drug Wegovy. It has also added a second contract manufacturer to mitigate the shortage.
Generic giant Teva is planning to move away from generics and focus on developing complex, high-value products.
The US Supreme Court has turned down Amgen’s bid to revive the patents for cholesterol-lowering drug Repatha in its dispute with Sanofi and Regeneron. Separately, Johnson & Johnson has settled its lawsuit with Amgen that will allow the latter to launch its Stelara biosimilar by January 1, 2025.
In approvals, AbbVie and Genmab have received the US Food and Drug Administration’s accelerated approval for their blood cancer therapy – Epkinly (epcoritamab) – to treat patients with diffuse large B-cell lymphoma (DLBCL). FDA has also granted approval to Krystal Biotech’s Vyjuvek, making it the first-ever topical gene therapy for the treatment of a rare genetic skin disorder. The agency also approved two drugs for opioid use disorder.
FDA has issued a Form 483 with four observations to Aurobindo Pharma’s active pharmaceutical ingredient (API) manufacturing facility in Andhra Pradesh (India). An FDA advisory panel has voted against the approval of Intercept Pharmaceuticals’ experimental treatment for nonalcoholic steatohepatitis (NASH).
In M&A news, Massachusetts-based Ironwood Pharmaceuticals is set to acquire rare disease drug developer VectivBio in a US$ 1.15 billion deal.
Due to soaring demand, Novo halts promotion of popular weight-loss drug Wegovy
There is an apparent gold rush for weight-loss drugs. The demand has been so high that Novo Nordisk has had to temporarily halt all television advertising campaigns for its blockbuster weight loss drug Wegovy (semaglutide, also sold under brand name Ozempic). Novo has also added a second contract manufacturer to mitigate the shortage.
Meanwhile, the pill version of Wegovy has shown promising results in a late-stage trial, leading to around 15.1 percent weight loss in patients who received a 50 mg daily dose for 68 weeks. A study conducted in the US has shown that Wegovy not only facilitates weight loss but also reduces the risk of heart disease. Similarly, Pfizer’s oral drug danuglipron has shown weight loss comparable to Novo’s Ozempic injection in a phase 2 clinical trial in adults with type 2 diabetes. The drug is also being studied in a phase 2b trial for adults with obesity.
Meanwhile, a Reuters report says the enormous demand for weight-loss treatments like Wegovy could support as many as 10 competing products with annual sales reaching up to US$ 100 billion within a decade, mostly in the US. Companies like Amgen (AMG133), Pfizer and Sosei Heptares (PF-07081532), Altimmune (pemvidutide), Viking Therapeutics (VK2735) and Zealand Pharma and Boehringer Ingelheim (BI 456906) are working on treatments for obesity, while Eli Lilly’s type 2 diabetes drug Mounjaro is likely to bag FDA approval for weight loss sometime this year.
Novo in pact with Life Edit Therapeutics: Novo Nordisk and Life Edit Therapeutics, an ElevateBio company focused on next-generation gene editing technologies and therapeutics, have announced a research and development collaboration to discover and develop gene editing therapies for rare and cardio-metabolic diseases.
FTC probes Abbott, other baby formula makers for collusion in state contract bids
The US Federal Trade Commission (FTC) is investigating whether Abbott Laboratories and other baby formula companies colluded in bidding for state contracts. FTC had initiated the investigation in 2022 and Abbott has said it is cooperating with FTC’s requests for information. FTC has also sought information from Nestlé.
Teva to cut down generic production; to shift focus to high-value products
Generic giant Teva is planning to cut back on the production of some generic drugs, citing low profits. The Israeli drugmaker said it will discontinue certain older generics that are already being supplied by multiple other manufacturers and reduce the development of new generics as it shifts its focus on developing complex, high-value products. Teva assured it will not drop drugs that are currently in high demand and experiencing shortages.
US Supreme Court rejects Amgen’s bid to revive cholesterol drug patents
In a major setback to Amgen, the US Supreme Court has ruled against the California-based drugmaker by rejecting an attempt to revive the patents for its cholesterol-lowering drug Repatha in its legal dispute with rivals Sanofi and Regeneron.
J&J, Amgen settle lawsuit over Stelara’s biosim: Johnson & Johnson has settled its lawsuit with Amgen over the latter’s proposed biosimilar of its blockbuster psoriasis and autoimmune diseases drug Stelara. The settlement will allow Amgen to launch its Stelara biosimilar “no later than January 1st, 2025”.
Amgen’s Horizon takeover delayed: Amgen said it will delay its US$ 27.8 billion acquisition of Horizon Therapeutics after the FTC filed a lawsuit to prevent the deal. A district court issued a temporary restraining order following an agreement between Amgen, Horizon and FTC.
FDA grants accelerated approval to AbbVie, Genmab’s blood cancer therapy
AbbVie and Danish drugmaker Genmab have received FDA’s accelerated approval for their blood cancer therapy – Epkinly (epcoritamab) – to treat patients with diffuse large B-cell lymphoma (DLBCL) who have undergone at least two lines of systemic therapy. Epkinly is the first-of-its-kind therapy to be approved for DLBCL, a type of cancer that originates in white blood cells.
Meanwhile, AbbVie’s blockbuster drug Rinvoq has received its seventh FDA approval – this time to treat patients with moderate to severe Crohn’s disease who have had an inadequate response or intolerance to at least one TNF blocker.
Approves Krystal’s gene therapy for rare genetic skin disorder: FDA has granted approval to Krystal Biotech’s Vyjuvek, making it the first-ever topical gene therapy for the treatment of dystrophic epidermolysis bullosa (DEB), a rare genetic skin disorder.
Blueprint Medicines’ Ayvakit has received FDA’s expanded approval as the first med to treat indolent systemic mastocytosis (SM), a condition characterized by an excessive build-up of mast cells in the body. The drug was first approved in 2021 for an advanced form of the disease.
FDA has also approved Xacduro, a treatment for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia.
Opioid use disorder drugs bag approval: Opiant Pharmaceuticals’ nasal spray Opvee (nalmefene) has received FDA approval for the reversal of opioid-related overdoses in individuals aged 12 years and older. FDA has also granted approval to Braeburn’s long-acting Brixadi to treat opioid use disorder.
FDA panel votes against approval of Intercept’s fatty liver drug
A panel of external advisers to the FDA has voted against the approval of Intercept Pharmaceuticals’ experimental oral drug – obeticholic acid – for the treatment of nonalcoholic steatohepatitis (NASH), a type of fatty liver disease.
FDA’s Form 483 for Aurobindo’s API unit: FDA has issued a Form 483 with four observations to Aurobindo Pharma’s (Unit XIV) API facility at Paravada Industrial Area in Andhra Pradesh (India). The agency carried out an inspection of the non-antibiotic manufacturing facility from May 15 to 19.
Ironwood to acquire rare disease drug developer VectivBio for US$ 1.15 billion
Massachusetts-based drugmaker Ironwood Pharmaceuticals is set to acquire rare disease drug developer VectivBio in a US$ 1.15 billion all-cash deal. The deal will give Ironwood, which specializes in stomach and intestinal diseases, the rights to VectivBio’s lead drug apraglutide, which is currently in late-stage testing for short bowel syndrome with intestinal failure.
Meanwhile, bankrupt Purdue Pharma has received a US court’s approval to sell its consumer health business Avrio to Atlantis Consumer Healthcare, a subsidiary of Arcadia Consumer Healthcare, for US$ 397 million.The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Phisper Infographic by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”