
Synopsis
0
USDMF
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
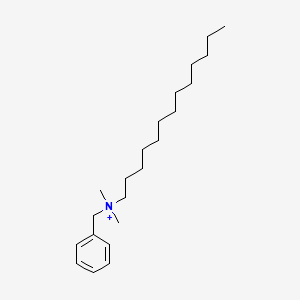

1. Alkyldimethylbenzylammonium Chloride
2. Asepsol
3. Benzalkonium Chloride
4. Benzalkonium Compounds
5. Btc 2125
6. Btc-2125
7. Btc2125
8. Chloride, Alkyldimethylbenzylammonium
9. Chloride, Benzalkonium
10. Compounds, Benzalkonium
11. Drapolene
12. Germex
13. Osvan
14. Zephiran
1. 47309-59-1
2. Benzyldimethyltridecylammonium
3. N-benzyl-n,n-dimethyltridecan-1-aminium
4. Benzyl-dimethyl-tridecylazanium
5. Spbio_001472
6. Benzalkonium Ion
7. Benzalkonium Cation
8. Spectrum_001442
9. Spectrum2_001566
10. Spectrum3_001597
11. Spectrum4_000861
12. Spectrum5_001097
13. Benzyldimethyltridecylaminium
14. Bspbio_003194
15. Kbiogr_001521
16. Kbioss_001922
17. Divk1c_000825
18. Schembl7125738
19. Chembl1187417
20. Kbio1_000825
21. Kbio2_001922
22. Kbio2_004490
23. Kbio2_007058
24. Kbio3_002694
25. Chebi:188978
26. Ninds_000825
27. Zinc8214735
28. Idi1_000825
29. Qtl1_000011
30. Ncgc00178214-01
31. Ncgc00178214-02
32. Ncgc00178214-04
33. Sbi-0051833.p002
34. Ft-0622628
35. Ab00053823_02
| Molecular Weight | 318.6 g/mol |
|---|---|
| Molecular Formula | C22H40N+ |
| XLogP3 | 7.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 14 |
| Exact Mass | 318.316075280 g/mol |
| Monoisotopic Mass | 318.316075280 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 1 |
| Complexity | 253 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
When used as an active ingredient in products like antibacterial, antiseptic, or disinfectant soaps, topical sanitizers, or cleaning agents, benzalkonium is primarily implemented in its salt form, benzalkonium chloride, where it may often be the only active ingredient present and indicated for the primary purpose of topical washing to decrease bacteria on skin. Conversely, when implemented as an excipient ingredient in a variety of multidose aqueous nose, eye, or ear products, benzalkonium chloride is being used as the antimicrobial preservative of choice to facilitate effective bactericidal and fungicidal actions to help minimize the growth of unwanted organisms in the multidose containers.
FDA Label
Benzalkonium chloride solutions are generally categorized as biocidal agents with relative long durations of action. Their spectrum of activity has been demonstrated against bacteria, to some viruses, fungi, and protozoa, although bacterial spores are treated as being resistant to the agent. Additionally, the agent generally shows more activity against gram-positive than gram-negative bacteria. Finally, solutions of benzalkonium chloride are bacteriostatic or bactericidal based on their concentration. Bacteriostatic agents act to prevent further growth of bacterial organisms that are present while bactericidal agents function to kill bacteria that are present. In general, the activity of the agent is not largely affected by pH, but such activity does increase substantially at higher temperatures and prolonged exposure times.
Preservatives, Pharmaceutical
Substances added to pharmaceutical preparations to protect them from chemical change or microbial action. They include ANTI-BACTERIAL AGENTS and antioxidants. (See all compounds classified as Preservatives, Pharmaceutical.)
Detergents
Purifying or cleansing agents, usually salts of long-chain aliphatic bases or acids, that exert cleansing (oil-dissolving) and antimicrobial effects through a surface action that depends on possessing both hydrophilic and hydrophobic properties. (See all compounds classified as Detergents.)
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AJ - Quaternary ammonium compounds
D08AJ01 - Benzalkonium
D - Dermatologicals
D09 - Medicated dressings
D09A - Medicated dressings
D09AA - Medicated dressings with antiinfectives
D09AA11 - Benzalkonium
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AA - Antiseptics
R02AA16 - Benzalkonium
Absorption
Percutaneous absorption is considered to be insignificant. In one study, benzalkonium chloride absorption was evaluated in women using tampons containing the agent. Venous blood samples were drawn 15 minutes before the tampon application and then again at 15 min, 1 h, 3 h, and 24 h after application. Benzalkonium chloride was not detected in any of the blood samples at any time tested. Similarly, in another study, benzalkonium chloride absorption was tested in women using tampons containing the agent. Venous blood and breast milk samples were taken 15 minutes before application and 3 h and 24 h after tampon administration. Benzalkonium chloride was not found in any of the subjects' samples.. Moreover, in a study where benzalkonium chloride solution was placed on the corneal surface of rabbit subjects, at various intervals after administration, the rabbits' eyes would be washed with 1 mL saline and the following tissues and fluids were removed: bulbar and palpebral conjunctiva, aqueous humour, corneal epithelium, endothelium and stroma, iris-ciliary body, lens, vitreous, retina, and choroid. Plasma samples were obtained with direct cardiac punctures. After administration of one drop, benzalkonium chloride was found in the corneal epithelium, endothelium, and stroma, and in the bulbar and palpebral conjunctivae. Benzalkonium chloride loss from ocular tissues was such that about one-third to two thirds of its concentration (depending on the tissue) at 30 min remained after 24 hr; measurable values existed for as long as 120 hr. The administration of multiple drops led to continued accumulation of benzalkonium chloride..
Route of Elimination
Administered benzalkonium chloride is likely eliminated largely in faeces, similar to other quaternary ammonium compounds.
Volume of Distribution
When applied as a topical antibacterial, antiseptic, disinfectant, or sanitizer it is believed that molecules of benzalkonium chloride are poorly absorbed (perhaps due to their large, positively charged nature), especially considering expectations for such topical applications to keep their biocidal agents available for action at the topical level and to not be absorbed significantly beyond it. When benzalkonium chloride is implemented as an excipient preservative ingredient in various eye, nose, and ear aqueous products, such products will always have other active pharmacological agents whose volume of distribution will be of greater importance. In these cases the excipients will only ever be present at the minimal levels necessary to maintain the integrity of the product substance. Moreover, Benzalkonium chloride is currently listed as a Category III ingredient by the United States Food and Drug Administration. Ingredients are listed in the FDA Category III when the data available about them are insufficient to classify as safe and effective, requiring further testing to determine more formal details about elements like human pharmacokinetic studies, and studies on the ingredients' absorption, distribution, metabolism, and excretion.
Since benzalkonium chloride is structurally a large, positively charged molecule it is likely poorly absorbed and eliminated largely in faeces, similar to other quaternary ammonium compounds.
Although not entirely elucidated, the bactericidal action of benzalkonium chloride is believed to be due to the disruption of intermolecular interactions. Such disruption can cause the dissociation of cellular membrane lipid bilayers of bacteria, resulting in compromised cellular permeability control and the leakage of important cellular contents. Additionally, other important molecular complexes like enzymes which control the maintenance of a great range of respiratory and metabolic cellular activities, are also susceptible to such deactivation. Consequently, a variety of critical intermolecular interactions and tertiary structures in very highly specific biochemical systems that allow bacterial agents to function normally can be readily disrupted or deactivated by cationic surfactants like benzalkonium chloride..

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ANALYTICAL

ABOUT THIS PAGE
13
PharmaCompass offers a list of Benzalkonium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Benzalkonium manufacturer or Benzalkonium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Benzalkonium manufacturer or Benzalkonium supplier.
PharmaCompass also assists you with knowing the Benzalkonium API Price utilized in the formulation of products. Benzalkonium API Price is not always fixed or binding as the Benzalkonium Price is obtained through a variety of data sources. The Benzalkonium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Benzalkonium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Benzalkonium, including repackagers and relabelers. The FDA regulates Benzalkonium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Benzalkonium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Benzalkonium supplier is an individual or a company that provides Benzalkonium active pharmaceutical ingredient (API) or Benzalkonium finished formulations upon request. The Benzalkonium suppliers may include Benzalkonium API manufacturers, exporters, distributors and traders.
click here to find a list of Benzalkonium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Benzalkonium CEP of the European Pharmacopoeia monograph is often referred to as a Benzalkonium Certificate of Suitability (COS). The purpose of a Benzalkonium CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Benzalkonium EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Benzalkonium to their clients by showing that a Benzalkonium CEP has been issued for it. The manufacturer submits a Benzalkonium CEP (COS) as part of the market authorization procedure, and it takes on the role of a Benzalkonium CEP holder for the record. Additionally, the data presented in the Benzalkonium CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Benzalkonium DMF.
A Benzalkonium CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Benzalkonium CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Benzalkonium suppliers with CEP (COS) on PharmaCompass.
A Benzalkonium written confirmation (Benzalkonium WC) is an official document issued by a regulatory agency to a Benzalkonium manufacturer, verifying that the manufacturing facility of a Benzalkonium active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Benzalkonium APIs or Benzalkonium finished pharmaceutical products to another nation, regulatory agencies frequently require a Benzalkonium WC (written confirmation) as part of the regulatory process.
click here to find a list of Benzalkonium suppliers with Written Confirmation (WC) on PharmaCompass.
Benzalkonium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Benzalkonium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Benzalkonium GMP manufacturer or Benzalkonium GMP API supplier for your needs.
A Benzalkonium CoA (Certificate of Analysis) is a formal document that attests to Benzalkonium's compliance with Benzalkonium specifications and serves as a tool for batch-level quality control.
Benzalkonium CoA mostly includes findings from lab analyses of a specific batch. For each Benzalkonium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Benzalkonium may be tested according to a variety of international standards, such as European Pharmacopoeia (Benzalkonium EP), Benzalkonium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Benzalkonium USP).
