
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
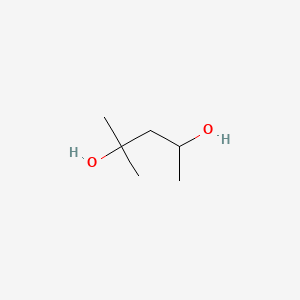

1. 2-methyl-2,4-pentanediol
2. 2-methylpentane-2,4-diol
3. Hexylene Glycol, Titanium(4+) Salt
1. 2-methyl-2,4-pentanediol
2. 107-41-5
3. 2-methylpentane-2,4-diol
4. Diolane
5. 2,4-pentanediol, 2-methyl-
6. 2,4-dihydroxy-2-methylpentane
7. Isol
8. Pinakon
9. 4-methyl-2,4-pentanediol
10. 1,1,3-trimethyltrimethylenediol
11. 2-methyl Pentane-2,4-diol
12. Hexyleneglycol
13. 2-methyl-2,4-pentandiol
14. Hexylene Glycol [nf]
15. Nsc-8098
16. Alpha,alpha,alpha'-trimethyltrimethylene Glycol
17. Keh0a3f75j
18. 1,3-dimethyl-3-hydroxybutanol
19. Chebi:62995
20. 1,3,3-trimethyl-1,3-propanediol
21. Mfcd00004547
22. Hexylene Glycol (nf)
23. Nsc 8098
24. Dsstox_cid_1885
25. Dsstox_rid_76384
26. Dsstox_gsid_21885
27. Caswell No. 574
28. Cas-107-41-5
29. 2-methylpentan-2,4-diol
30. Hsdb 1126
31. 2-methyl-pentane-2,4-diol
32. (+-)-2-methyl-2,4-pentanediol
33. Einecs 203-489-0
34. Unii-keh0a3f75j
35. Epa Pesticide Chemical Code 068601
36. Brn 1098298
37. Ai3-00919
38. Ccris 9439
39. Hexylene Glycol, 99%
40. R-(-)-2-methyl-2,4-pentanediol
41. 2methyl-2,4-pentanediol
42. 2-methyl-2-4-pentanediol
43. Ec 203-489-0
44. Hexylene Glycol, >=99%
45. Hexylene Glycol, 99.5%
46. Schembl19379
47. Hexylene Glycol [ii]
48. Hexylene Glycol [mi]
49. 1,3-trimethyltrimethylenediol
50. 4-01-00-02565 (beilstein Handbook Reference)
51. Hexylene Glycol [hsdb]
52. Hexylene Glycol [inci]
53. Chembl2104293
54. Dtxsid5021885
55. Hexylene Glycol [mart.]
56. Hexylene Glycol [usp-rs]
57. Nsc8098
58. (?)-2-methyl-2,4-pentanediol
59. Hms3264e19
60. 1,1,3-trimethyl-1,3-propanediol
61. Hy-b0903
62. Hexylene Glycol, Analytical Standard
63. Tox21_201975
64. Tox21_302818
65. (+/-)-2-methyl-2,4-pentanediol
66. S3588
67. Akos015901459
68. Ccg-213719
69. Wln: Qy1 & 1xq1 & 1
70. Ncgc00249143-01
71. Ncgc00256494-01
72. Ncgc00259524-01
73. (+/-)-2,4-dihydroxy-2-methyl Pentane
74. Ac-13749
75. As-58339
76. Hexylene Glycol, Bioxtra, >=99% (gc)
77. (+/-)-2-methyl-2,4-pentanediol, Mpd
78. (^+)-2-methyl-2,4-pentanediol, 98%
79. Db-057767
80. Ft-0605050
81. Ft-0605756
82. Ft-0613069
83. Hexylene Glycol, Puriss., >=99.0% (gc)
84. M0384
85. .alpha.,.alpha.'-trimethyltrimethylene Glycol
86. Hexylene Glycol, Bioultra, >=99.0% (gc)
87. D04439
88. Ab01563179_01
89. J-640306
90. J-660006
91. Q2792203
92. W-108748
93. Hexylene Glycol, United States Pharmacopeia (usp) Reference Standard
94. Hexylene Glycol, Pharmagrade, Usp/nf, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production
| Molecular Weight | 118.17 g/mol |
|---|---|
| Molecular Formula | C6H14O2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 118.099379685 g/mol |
| Monoisotopic Mass | 118.099379685 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 8 |
| Formal Charge | 0 |
| Complexity | 68.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Between toxicity ratings 2 or 3. 2= Slightly toxic: probable oral lethal dose (human) 5-15 g/kg, between 1 pint & 1 qt for 70 kg person (150 lb). 3= Moderately toxic: probable oral lethal dose (human) 0.5-5 g/kg; between 1 oz & 1 pint (or 1 lb) for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-179
... Mice /were fed hexylene glycol/ orally 20 mg/day in 2 mL of whole milk for up to 81 days ... . approximately 40% of the hexylene glycol was accounted for in the urine, but only 4% of the amount excreted was free glycol; the other 36% was conjugated with glycuronic acid.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 50
... Not readily absorbed through the skin ... .
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 49
Eliminated in urine, partly (20-25%) in conjugated forms.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-179
... Oral administration of hexylene glycol to rats & rabbits resulted in a substantial increase in the amt of hexuronates in the plasma & in the urine.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 50
For more Absorption, Distribution and Excretion (Complete) data for 2-METHYL-2,4-PENTANEDIOL (8 total), please visit the HSDB record page.
...Five human subjects ... /had/ both free & conjugated hexylene glycol in the urine after single or repeated oral doses. ...
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 50
(14)C-hexylene glycol fed to rabbits... urine contained 7 metabolites incl glucuronide of hexylene glycol (46% of dose), unchanged hexylene glycol (2.5%), diacetone alcohol (1.4%) & an unidentified glucuronide which could be conjugate of diacetone alcohol. ...Converted into diacetone alc by incubation with rat liver slices.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 166
... Oral administration of hexylene glycol to rats & rabbits resulted in a substantial increase in the amt of hexuronates in the plasma & in the urine.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 50
It was also shown that approximately 40% of the hexylene glycol was accounted for in the urine, but only 4% of the amount excreted was free glycol; the other 36% was conjugated with glycuronic acid.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 50
...after a single oral (gavage) administration of Hexylene glycol at the dose-level of 590 mg/kg to male Sprague-Dawley rats ...t1/2 21.2 hr
European Chemicals Agency (ECHA); REACH Registration for 2-methylpentane-2,4-diol (CAS 107-41-5); Available from, as of May 29, 2014: https://echa.europa.eu/
Some effects of gravity on early morphogenesis are correlated with microtubule locations within cells. During first cleavage in Ilyanassa obsoleta embryos, a transitory polar lobe constriction forms and then relaxes, allowing the polar lobe to merge with one daughter cell. If the polar lobe is equally divided or removed, morphogenesis is severely disrupted. To examine microtuble locations during early Ilyanassa development, eggs were fixed and stained for polymerized alpha-tubulin during first cleavage. The mitotic apparatus assembles at the animal pole. The cleavage furrow forms between the asters, constricting to a stabilized intercellular bridge encircling midbody-bound microtubules, whereas the polar lobe constriction forms below and parallel to the spindle, constricting to a transitory intercellular bridge encircling no detectable microtubules. At metaphase an alpha-tubulin epitope is distributed throughout the spindle, whereas a beta-tubulin epitope is present predominantly in the asters. Incubation in hexylene glycol, a drug that increases microtubule polymerization, during mitosis causes the polar lobe constriction to tighten around polymerized alpha-tubulin and remain stably constricted. If hexylene glycol is removed, alpha-tubulin staining disappears from the polar lobe constriction, which relaxes, whereas microtubules remain in the cleavage furrow, which remains constricted. These observations suggest that asymmetric distribution of microtubules affects early Ilyanassa cleavage patterns, and that continued presence of microtubules extending through an intercellular bridge is important for stabilization of the bridge constriction prior to completion of cytokinesis. These data provide the basis for further analysis of the role of microtubules in possible microgravity disruptions of Ilyanassa development.
PMID:11536633 Conrad AH et al; J Exp Zool 269 (3): 188-204 (1994)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Hexylene Glycol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hexylene Glycol manufacturer or Hexylene Glycol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hexylene Glycol manufacturer or Hexylene Glycol supplier.
PharmaCompass also assists you with knowing the Hexylene Glycol API Price utilized in the formulation of products. Hexylene Glycol API Price is not always fixed or binding as the Hexylene Glycol Price is obtained through a variety of data sources. The Hexylene Glycol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hexylene Glycol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hexylene Glycol, including repackagers and relabelers. The FDA regulates Hexylene Glycol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hexylene Glycol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hexylene Glycol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hexylene Glycol supplier is an individual or a company that provides Hexylene Glycol active pharmaceutical ingredient (API) or Hexylene Glycol finished formulations upon request. The Hexylene Glycol suppliers may include Hexylene Glycol API manufacturers, exporters, distributors and traders.
click here to find a list of Hexylene Glycol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Hexylene Glycol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hexylene Glycol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hexylene Glycol GMP manufacturer or Hexylene Glycol GMP API supplier for your needs.
A Hexylene Glycol CoA (Certificate of Analysis) is a formal document that attests to Hexylene Glycol's compliance with Hexylene Glycol specifications and serves as a tool for batch-level quality control.
Hexylene Glycol CoA mostly includes findings from lab analyses of a specific batch. For each Hexylene Glycol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hexylene Glycol may be tested according to a variety of international standards, such as European Pharmacopoeia (Hexylene Glycol EP), Hexylene Glycol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hexylene Glycol USP).
