
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
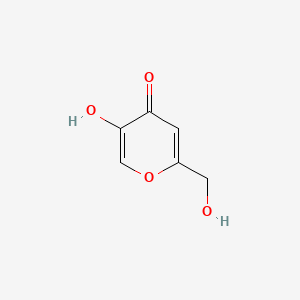

1. 5-((3-aminopropyl)phosphinooxy)-2-(hydroxymethyl)-4h-pyran-4-one
2. Kojyl-appa
1. 501-30-4
2. 5-hydroxy-2-(hydroxymethyl)-4h-pyran-4-one
3. 5-hydroxy-2-(hydroxymethyl)-4-pyrone
4. 5-hydroxy-2-(hydroxymethyl)pyran-4-one
5. 5-hydroxy-2-hydroxymethyl-4-pyrone
6. 4h-pyran-4-one, 5-hydroxy-2-(hydroxymethyl)-
7. Acido Kojico
8. 2-hydroxymethyl-5-hydroxy-gamma-pyrone
9. 2-(hydroxymethyl)-5-hydroxy-4h-pyran-4-one
10. Mfcd00006580
11. 5-hydroxy-2-hydroxymethyl-4h-4-pyranone
12. 5-hydroxy-2-hydroxymethyl-pyran-4-one
13. 5-hydroxy-2-hydroxymethyl-4h-pyran-4-one
14. Pyran-4-one, 5-hydroxy-2-(hydroxymethyl)
15. Chembl287556
16. 123712-78-7
17. Chebi:43572
18. Nsc1942
19. 6k23f1tt52
20. Nsc-1942
21. Ncgc00017325-03
22. Ncgc00017325-06
23. Dsstox_cid_20236
24. Dsstox_rid_79455
25. Dsstox_gsid_40236
26. Cas-501-30-4
27. Ccris 4131
28. Nsc 1942
29. Einecs 207-922-4
30. Brn 0120895
31. Kojisaeure
32. Unii-6k23f1tt52
33. Ai3-02549
34. Hsdb 7664
35. Kojic Acid Solution
36. Spectrum_000191
37. Kojic Acid [mi]
38. Spectrum2_001828
39. Spectrum3_001704
40. Spectrum4_000571
41. Spectrum5_001085
42. Natural Kojic Acid Powder
43. Kojic Acid [hsdb]
44. Kojic Acid [iarc]
45. Kojic Acid [inci]
46. Kojic Acid Dipalmitate 5kg
47. Kojic Acid [mart.]
48. Oprea1_038773
49. Schembl36895
50. Bspbio_003288
51. Kbiogr_001002
52. Kbioss_000671
53. Kojic Acid [who-dd]
54. 5-18-02-00516 (beilstein Handbook Reference)
55. Bidd:er0501
56. Divk1c_000923
57. Spbio_001875
58. Kojic Acid, Analytical Standard
59. Megxm0_000388
60. Wln: T6o Dvj B1q Eq
61. Dtxsid2040236
62. Acon1_000622
63. Bejnerdrqowkjm-uhfffaoysa-
64. Hms502o05
65. Kbio1_000923
66. Kbio2_000671
67. Kbio2_003239
68. Kbio2_005807
69. Kbio3_002508
70. Ninds_000923
71. Hms3604l04
72. Kuc106760n
73. Tnp00261
74. 2-hydroxymethyl-5-hydroxy-?-pyrone
75. 2-hydroxymethyl-5-hydroxy-g-pyrone
76. Tox21_110814
77. Tox21_113449
78. Bbl010645
79. Bdbm50031467
80. Ccg-38458
81. S5174
82. Stk801688
83. Zinc13831818
84. Akos000120649
85. Tox21_110814_1
86. 5-hydroxy-2-hydroxymethyl-i(3)-pyron
87. Db01759
88. Hy-w050154
89. Idi1_000923
90. Smp1_000171
91. Ncgc00017325-01
92. Ncgc00017325-02
93. Ncgc00017325-04
94. Ncgc00017325-05
95. Ncgc00017325-10
96. Ncgc00142361-01
97. Ncgc00168903-01
98. Ncgc00168903-02
99. Ncgc00181145-01
100. Ac-11658
101. As-11648
102. Kojic Acid, Purum, >=98.0% (hplc)
103. Ksc-11-228-2
104. Sy018009
105. 2-hydroxymethyl-5-hydroxy-4-pyrone
106. 2-hydroxymethyl-5-hydroxy-4h-pyran-4-one
107. Cs-0032701
108. Ft-0627581
109. K0010
110. K0012
111. 01k304
112. C91105
113. K-7000
114. 2-hydroxymethyl-5-hydroxy-.gamma.-pyrone
115. Q416285
116. Sr-01000945134
117. Sr-01000945134-1
118. 5-hydroxy-2-(hydroxymethyl)-4h-pyran-4-one Solution
119. C65a72e0-0e46-4af4-aa68-178eca2e5fcc
120. F0001-1307
121. Z381040328
| Molecular Weight | 142.11 g/mol |
|---|---|
| Molecular Formula | C6H6O4 |
| XLogP3 | -0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 142.02660867 g/mol |
| Monoisotopic Mass | 142.02660867 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 10 |
| Formal Charge | 0 |
| Complexity | 214 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Depigmenting agent /for skin lightening/
PMID:18369335 Zhu W, Gao J; J Investig Dermatol Symp Proc 13 (1): 20-4 (2008)
Melasma is a chronic and recurrent disorder. It has been underdiagnosed and undertreated due to lack of effective therapies and the perception that it is merely a cosmetic nuisance. Hydroquinone, corticosteroids, licorice extracts and kojic acid have been used as monotherapy to treat melasma. However, the present standard of care in melasma therapy is combination therapy. To date, the most effective treatment is a triple-combination agent that contains hydroquinone 4%, tretinoin 0.05% and fluocinolone acetonide 0.01%...
Rendon MI; J Drugs Dermatol (5 Suppl):S27-34 (2004)
... Combination regimens, including frequent applications of superficial- and medium-depth chemical peels, appear to be particularly effective and well tolerated in dark-skinned patients with melanosis. Post-inflammatory hyperpigmentation is the result of excess pigment deposition following an inflammatory skin disorder. Topical tretinoin, hydroquinone, azelaic acid, kojic acid, and glycolic acid peels have been employed with variable degrees of success...
PMID:15186195 Stratigos AJ, Katsambas A; Am J Clin Dermatol 5 (3): 161-8 (2004)
Facial and neck pigmentations are ... common in middle-aged women, and are related to endogenous (hormones) and exogenous factors (such as use of cosmetics and perfumes, and exposure to sun radiation). Melasma (chloasma) is the most common cause of facial pigmentation, but there are many other forms such as Riehl's melanosis, poikiloderma of Civatte, erythrose peribuccale pigmentaire of Brocq, erythromelanosis follicularis of the face and neck, linea fusca, and cosmetic hyperpigmentations. Treatment of melasma and other facial pigmentations has always been challenging and discouraging.... Several hypopigmenting agents have been used with differing results. Topical hydroquinone 2 to 4% alone or in combination with tretinoin 0.05 to 0.1% is an established treatment. Topical azelaic acid 15 to 20% can be as efficacious as hydroquinone, but is less of an irritant. Tretinoin is especially useful in treating hyperpigmentation of photoaged skin. Kojic acid, alone or in combination with glycolic acid or hydroquinone, has shown good results, due to its inhibitory action on tyrosinase. Chemical peels are useful to treat melasma: trichloroacetic acid, Jessner's solution, Unna's paste, alpha-hydroxy acid preparations, kojic acid, and salicyclic acid, alone or in various combinations have shown good results. In contrast, laser therapies have not produced completely satisfactory results, because they can induce hyperpigmentation and recurrences can occur. New laser approaches could be successful at clearing facial hyperpigmentation in the future.
PMID:11702317 Perez-Bernal A et al; Am J Clin Dermatol 1 (5): 261-8 (2000)
For more Therapeutic Uses (Complete) data for KOJIC ACID (7 total), please visit the HSDB record page.
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
The structure of kojic acid indicates a relatively simple route of metabolism much like dietary hexoses.
PMID:11259181 Burdock GA et al; Regul Toxicol Pharmacol 33 (1): 80-101 (2001)
The mechanism of action of kojic acid is well defined and it has been shown to act as a competitive and reversible inhibitor of animal and plant polyphenol oxidases, xanthine oxidase, and D- and some L-amino acid oxidases.
PMID:11259181 Burdock GA et al; Regul Toxicol Pharmacol 33 (1): 80-101 (2001)
The activation of NF-kappaB induced by kojic acid, an inhibitor of tyrosinase for biosynthesis of melanin in melanocytes, was investigated in human transfectant HaCaT and SCC-13 cells. These two keratinocyte cell lines transfected with pNF-kappaB-SEAP-NPT plasmid were used to determine the activation of NF-kappaB. Transfectant cells release the secretory alkaline phosphatase (SEAP) as a transcription reporter in response to the NF-kappaB activity and contain the neomycin phosphotransferase (NPT) gene for the dominant selective marker of geneticin resistance. NF-kappaB activation was measured in the SEAP reporter gene assay using a fluorescence detection method. Kojic acid showed the inhibition of cellular NF-kappaB activity in both human keratinocyte transfectants. It could also downregulate the ultraviolet ray (UVR)-induced activation of NF-kappaB expression in transfectant HaCaT cells. Moreover, the inhibitory activity of kojic acid in transfectant HaCaT cells was found to be more potent than known antioxidants, e.g., vitamin C and N-acetyl-L-cysteine. These results indicate that kojic acid is a potential inhibitor of NF-kappaB activation in human keratinocytes, and suggest the hypothesis that NF-kappaB activation may be involved in kojic acid induced anti-melanogenic effect.
Moon KY et al; Arch Pharm Res 24 (4): 307-11 (2001):
Market Place

Reply
22 Nov 2019
Reply
27 Aug 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE
81
PharmaCompass offers a list of Kojic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Kojic Acid manufacturer or Kojic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Kojic Acid manufacturer or Kojic Acid supplier.
PharmaCompass also assists you with knowing the Kojic Acid API Price utilized in the formulation of products. Kojic Acid API Price is not always fixed or binding as the Kojic Acid Price is obtained through a variety of data sources. The Kojic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Kojic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Kojic Acid, including repackagers and relabelers. The FDA regulates Kojic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Kojic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Kojic Acid supplier is an individual or a company that provides Kojic Acid active pharmaceutical ingredient (API) or Kojic Acid finished formulations upon request. The Kojic Acid suppliers may include Kojic Acid API manufacturers, exporters, distributors and traders.
Kojic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Kojic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Kojic Acid GMP manufacturer or Kojic Acid GMP API supplier for your needs.
A Kojic Acid CoA (Certificate of Analysis) is a formal document that attests to Kojic Acid's compliance with Kojic Acid specifications and serves as a tool for batch-level quality control.
Kojic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Kojic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Kojic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Kojic Acid EP), Kojic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Kojic Acid USP).
