
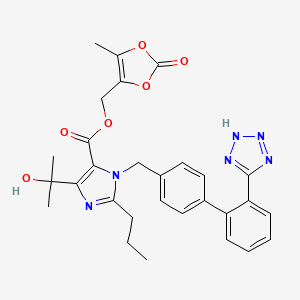

1. 5-methyl-2-oxo-1,3-dioxolen-4-yl)methoxy-4-(1-hydroxy-1-methylethyl)-2-propyl-1-(4-(2-(tetrazol-5-yl)phenyl)phenyl)methylimidazol-5-carboxylate - T287346
2. Benicar
3. Cs 866
4. Cs-866
5. Cs866
6. Medoxomil, Olmesartan
7. Olmetec
8. Votum
1. 144689-63-4
2. Benicar
3. Olmetec
4. Cs-866
5. Cs 866
6. Nsc-758924
7. 6m97xtv3hd
8. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylate
9. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester
10. Ncgc00095136-01
11. Benevas
12. Olsertain
13. Olvance
14. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
15. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
16. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(2h-tetrazol-5-yl)biphenyl-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
17. Benicar (tn)
18. Olmetec (tn)
19. Cs866
20. Sr-05000001987
21. Unii-6m97xtv3hd
22. Votume
23. Openvas
24. Cs-866dm
25. Cs-866rn
26. Olmesartanmedoxomil
27. Olmesartan-medoxomil
28. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(1h-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylate
29. Olmesartan Medoxomil [usan:inn:ban]
30. Ks-1182
31. Olmesartan (medoxomil)
32. Spectrum_001944
33. Spectrum2_000506
34. Spectrum3_001676
35. Spectrum4_000740
36. Spectrum5_001556
37. Dsstox_cid_25924
38. Dsstox_rid_81226
39. Dsstox_gsid_45924
40. Schembl16403
41. Bspbio_003491
42. Gtpl591
43. Kbiogr_001040
44. Kbioss_002498
45. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl) (1,1'-biphenyl)-4-yl)methyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl) Methyl Ester
46. Mls006010109
47. Spectrum1505205
48. Spbio_000431
49. Olmesartan Medoxomil (benicar)
50. Chembl1200692
51. Dtxsid9045924
52. Chebi:31932
53. Kbio2_002490
54. Kbio2_005058
55. Kbio2_007626
56. Kbio3_002711
57. Hms1922l15
58. Hms2089k18
59. Hms2093k16
60. Hms3651e13
61. Hms3715n09
62. Hms3743o09
63. Olmesartan Medoxomil (jp17/usp)
64. Olmesartan Medoxomil [inn]
65. Olmesartan Medoxomil [jan]
66. Pharmakon1600-01505205
67. Olmesartan Medoxomil [usan]
68. Amy22222
69. Bcp05214
70. Zinc4149248
71. Olmesartan Medoxomil [vandf]
72. Tox21_111445
73. Bdbm50442892
74. Ccg-39596
75. Mfcd00944911
76. Nsc758924
77. Olmesartan Medoxomil [mart.]
78. S1604
79. Stl451024
80. Olmesartan Medoxomil [usp-rs]
81. Olmesartan Medoxomil [who-dd]
82. Akos015894907
83. Akos015914772
84. Ac-1601
85. Bcp9000555
86. Ccg-221184
87. Cs-0577
88. Nsc 758924
89. Olmesartan Medoxomil, >=98% (hplc)
90. Sb19327
91. Ncgc00095136-02
92. Ncgc00095136-03
93. Ncgc00095136-09
94. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
95. Azor Component Olmesartan Medoxomil
96. Hy-17005
97. Olmesartan Medoxomil [orange Book]
98. Smr002203616
99. Olmesartan Medoxomil [ep Monograph]
100. Sbi-0206741.p001
101. Olmesartan Medoxomil [usp Monograph]
102. Cas-144689-63-4
103. Ft-0601603
104. O0510
105. Olmesartan Medoxomil Component Of Azor
106. Sw199650-2
107. D01204
108. Tribenzor Component Olmesartan Medoxomil
109. Ab01275443-01
110. Ab01275443_02
111. Ab01275443_03
112. Benicar Hct Component Olmesartan Medoxomil
113. 689o634
114. A808260
115. L001061
116. Olmesartan Medoxomil Component Of Tribenzor
117. J-501595
118. Olmesartan Medoxomil Component Of Benicar Hct
119. Sr-05000001987-1
120. Sr-05000001987-2
121. Brd-k78485176-001-02-9
122. Brd-k78485176-001-03-7
123. Q27888058
124. Z1550675457
125. Olmesartan Medoxomil, European Pharmacopoeia (ep) Reference Standard
126. Olmesartan Medoxomil, United States Pharmacopeia (usp) Reference Standard
127. Olmesartan Medoxomil For System Suitability, European Pharmacopoeia (ep) Reference Standard
128. Olmesartan Medoxomil, Pharmaceutical Secondary Standard; Certified Reference Material
129. (5-methyl-2-oxidanylidene-1,3-dioxol-4-yl)methyl 5-(2-oxidanylpropan-2-yl)-2-propyl-3-[[4-[2-(2h-1,2,3,4-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylate
130. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-i Midazole-5-carboxylate
131. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-((2'-(1h-tetrazol-5-yl)biphenyl-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
132. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 1-[[2'-(1h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl]-4-(2-hydroxypr
133. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxy-2-propanyl)-2-propyl-1-[[2'-(1h-tetrazol-5-yl)-4-biphenylyl]methyl]-1h-imidazole-5-carboxylate
134. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl}-1h-imidazole-5-carboxylate
135. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl1-((2'-(2h-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-4-(2-hydroxypropan-2-yl)-2-propyl-1h-imidazole-5-carboxylate
136. (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(1h-tetrazol-5-yl)biphenyl-4-yl]methyl]imidazole-5-carboxylate
137. (5-methyl-2-oxo-1,3-dioxolen-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(tetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylate
138. (5-methyl-2-oxo-1,3-dioxolen-4yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{4-[2-(tetrazol-5-yl)phenyl]phenyl}methylimidazole-5-carboxylate
139. (5-methyl-2-oxo-2h-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1h-1,2,3,4-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}-1h-imidazole-5-carboxylate
140. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-((2'-(1h-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, (5-methyl-2-oxo-1,3- Dioxol-4-yl)methyl Ester
141. 1h-imidazole-5-carboxylic Acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester
142. 5-(1-hydroxy-1-methylethyl)-2-propyl-3-[2'-(1h-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3h-imidazole-4-carboxylic Acid 5-methyl-2-oxo-[1,3]dioxol-4-ylmethyl Ester
143. 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2h-tetrazol-5-yl)phenyl]phenyl]methyl]-4-imidazolecarboxylic Acid (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester
144. Olmesartan Medoxomil
145. 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2h-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1h-imidazole-5-carboxylic Acid-(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester
| Molecular Weight | 558.6 g/mol |
|---|---|
| Molecular Formula | C29H30N6O6 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 558.22268270 g/mol |
| Monoisotopic Mass | 558.22268270 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 969 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | OLMESARTAN MEDOXOMIL |
| Active Ingredient | OLMESARTAN MEDOXOMIL |
| Company | ACCORD HLTHCARE (Application Number: A207662); ALEMBIC PHARMS LTD (Application Number: A203012); ALKEM LABS LTD (Application Number: A206763); AUROBINDO PHARMA LTD (Application Number: A204798); GLENMARK PHARMS LTD (Application Number: A203281); JUBILANT GENERICS (Application Number: A205482); LUPIN LTD (Application Number: A206631); MACLEODS PHARMS LTD (Application Number: A204814); MYLAN PHARMS INC (Application Number: A078276); TEVA PHARMS USA (Application Number: A091079); TORRENT PHARMS LTD (Application Number: A202375); ZYDUS PHARMS USA INC (Application Number: A205192) |
| 2 of 8 | |
|---|---|
| Drug Name | AZOR |
| Active Ingredient | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL |
| Company | DAIICHI SANKYO (Application Number: N022100) |
| 3 of 8 | |
|---|---|
| Drug Name | BENICAR HCT |
| Active Ingredient | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL |
| Company | DAIICHI SANKYO (Application Number: N021532) |
| 4 of 8 | |
|---|---|
| Drug Name | TRIBENZOR |
| Active Ingredient | AMLODIPINE BESYLATE; HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL |
| Company | DAIICHI SANKYO (Application Number: N200175) |
| 5 of 8 | |
|---|---|
| Drug Name | BENICAR |
| Active Ingredient | OLMESARTAN MEDOXOMIL |
| Company | DAIICHI SANKYO (Application Number: N021286) |
| 6 of 8 | |
|---|---|
| Drug Name | AMLODIPINE AND OLMESARTAN MEDOXOMIL |
| Active Ingredient | AMLODIPINE BESYLATE; OLMESARTAN MEDOXOMIL |
| Company | AJANTA PHARMA LTD (Application Number: A207216); ALEMBIC PHARMS LTD (Application Number: A207073); ALKEM LABS LTD (Application Number: A209042); AUROBINDO PHARMA LTD (Application Number: A206906); GLENMARK PHARMS LTD (Application Number: A207807); JUBILANT GENERICS (Application Number: A207450); MACLEODS PHARMS LTD (Application Number: A206884); MICRO LABS (Application Number: A207435); TEVA PHARMS USA (Application Number: A091154); TORRENT PHARMS LTD (Application Number: A202933); ZYDUS PHARMS USA INC (Application Number: A207771) |
| 7 of 8 | |
|---|---|
| Drug Name | OLMESARTAN MEDOXOMIL AND HYDROCHLOROTHIAZIDE |
| Active Ingredient | HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL |
| Company | ALEMBIC PHARMS LTD (Application Number: A204233); AUROBINDO PHARMA LTD (Application Number: A205391); MYLAN PHARMS INC (Application Number: A078827); PRINSTON INC (Application Number: A207804); TEVA PHARMS USA (Application Number: A200532); TORRENT PHARMS LTD (Application Number: A206515) |
| 8 of 8 | |
|---|---|
| Drug Name | OLMESARTAN MEDOXOMIL, AMLODIPINE AND HYDROCHLOROTHIAZIDE |
| Active Ingredient | AMLODIPINE BESYLATE; HYDROCHLOROTHIAZIDE; OLMESARTAN MEDOXOMIL |
| Company | PAR PHARM INC (Application Number: A206137); TEVA PHARMS USA (Application Number: A202491); TORRENT PHARMS LTD (Application Number: A203580) |
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
C09CA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA08 - Olmesartan medoxomil
BUILDING BLOCK

MARKET PLACE

