
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Erbumine, Perindopril
2. Perindopril Erbumine
3. Perstarium
4. Pirindopril
5. S 9490
6. S 9490 3
7. S 9490-3
8. S 94903
9. S-9490
10. S9490
1. 82834-16-0
2. S-9490
3. Perindoprilum
4. Mcn-a-2833
5. Coversyl
6. Chebi:8024
7. (2s,3as,7as)-1-[(2s)-2-[[(2s)-1-ethoxy-1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic Acid
8. Y5gmk36kgy
9. (2s,3as,7as)-1-[(2s)-2-{[(2s)-1-ethoxy-1-oxopentan-2-yl]amino}propanoyl]octahydro-1h-indole-2-carboxylic Acid
10. Dsstox_cid_3440
11. Dsstox_rid_77030
12. Dsstox_gsid_23440
13. (2s,3as,7as)-1-((s)-n-((s)-1-carboxybutyl)alanyl)hexahydro-2-indolinecarboxylic Acid, 1-ethyl Ester
14. Prestarium
15. Coverex
16. Coversum
17. Coverene Cor
18. Cas-82834-16-0
19. Perindoprilum [latin]
20. Unii-y5gmk36kgy
21. Perindopril (usan/inn)
22. Perindopril [usan:inn:ban]
23. Mcn-a 2833
24. S 9490
25. Ncgc00159509-02
26. Sed-9490
27. Dw-7950
28. Brn 4300272
29. Spectrum_001948
30. Perindopril [mi]
31. Spectrum2_001108
32. Spectrum3_001683
33. Spectrum4_000775
34. Spectrum5_001689
35. Perindopril [inn]
36. Perindopril [usan]
37. Perindopril [vandf]
38. Chembl1581
39. Perindopril [mart.]
40. Schembl16205
41. Bspbio_003206
42. Kbiogr_001190
43. Kbioss_002502
44. Perindopril [who-dd]
45. Mls002154153
46. Bidd:gt0786
47. Spbio_001216
48. Gtpl6367
49. Dtxsid6023440
50. Kbio2_002494
51. Kbio2_005062
52. Kbio2_007630
53. Kbio3_002426
54. Hms2098m04
55. Hms2232m24
56. Hms3715m04
57. Ex-a6377
58. Hy-b0130
59. Zinc3812867
60. Tox21_113087
61. Bdbm50493988
62. Akos025311315
63. Tox21_113087_1
64. Ccg-221101
65. Cs-1903
66. Db00790
67. Ncgc00274070-01
68. (2s)-2-[(1s)-1-carbethoxybutylamino]-1-oxopropyl-(2s,3as,7as)-perhydroindole-2-carboxylic Acid
69. (2s,3as,7as)-1-[(2s)-2-{[(2s)-1-ethoxy-1-oxopentan-2-yl]amino}propanoyl]-octahydro-1h-indole-2-carboxylic Acid
70. Ethyl N-{(2s)-1-[(2s,3as,7as)-2-carboxyoctahydro-1h-indol-1-yl]-1-oxopropan-2-yl}-l-norvalinate
71. Smr001233453
72. Sbi-0206736.p001
73. C07706
74. D03753
75. Ab00918721_06
76. 834p160
77. Q277785
78. S-90652
79. Sr-01000841817
80. J-523913
81. Sr-01000841817-2
82. Brd-k92731339-227-02-3
83. Brd-k92731339-227-03-1
84. (2s,3as,7as)-1-((s)-n-((s)-1-carboxybutyl)alanyl)hexahydro-2-indolinecarboxylicacid1-ethylester
85. (2s,3as,7as)-1-[(2s)-2-[[(2s)-1-ethoxy-1-oxo-2-pent-yl]amino]propanoyl]-octahydro-1h-indole-2-carboxylic Acid
86. (2s,3as,7as)-1-{(2s)-2-[(1s)-1-(ethoxycarbonyl)butylamino]propionyl}octahydro-1h-indole-2-carboxylic Acid
87. 11h-indole-2-carboxylic Acid, Octahydro-1-(2-((1-ethoxycarbonyl)butyl)amino)-1-oxopropyl)-, (2s-(1(r*(r*)),2-alpha,3a-beta,7a-beta))-
88. 1h-indole-2-carboxylic Acid, 1-(2-((1-(ethoxycarbonyl)butyl)amino)-1-oxopropyl)octahydro-, (2s-(1(r*(r*)),2.alpha.,3a.beta.,7a.beta.))-
89. 1h-indole-2-carboxylic Acid, 1-(2-((1-(ethoxycarbonyl)butyl)amino)-1-oxopropyl)octahydro-, (2s-(1(r*(r*)),2alpha,3abeta,7abeta))-
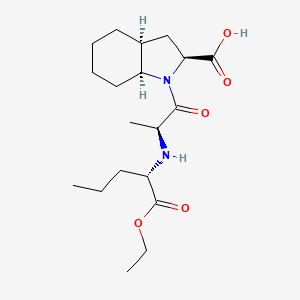
| Molecular Weight | 368.5 g/mol |
|---|---|
| Molecular Formula | C19H32N2O5 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 9 |
| Exact Mass | 368.23112213 g/mol |
| Monoisotopic Mass | 368.23112213 g/mol |
| Topological Polar Surface Area | 95.9 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 524 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of mild to moderate essential hypertension, mild to moderate congestive heart failure, and to reduce the cardiovascular risk of individuals with hypertension or post-myocardial infarction and stable coronary disease.
FDA Label
Perindopril is a nonsulfhydryl prodrug that is metabolized via first pass effect (62%) and systemic hydrolysis (38%) to perindoprilat, its active metabolite, following oral administration. Perindoprilat lowers blood pressure by antagonizing the effect of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure using a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium-potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may sustain the effects of perindoprilat by causing increased vasodilation and decreased blood pressure.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
C09AA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA04 - Perindopril
Absorption
Rapidly absorbed with peak plasma concentrations occurring approximately 1 hour after oral administration. Bioavailability is 65-75%. Following absorption, perindopril is hydrolyzed to perindoprilat, which has an average bioavailability of 20%. The rate and extent of absorption is unaffected by food. However, food decreases the extent of biotransformation to peridoprilat and reduces its bioavailability by 35%.
Route of Elimination
Perindopril is extensively metabolized following oral administration, with only 4 to 12% of the dose recovered unchanged in the urine.
Clearance
219 - 362 mL/min [oral administration]
Extensively metabolized, with only 4-12% of the dose recovered in urine following oral administration. Six metabolites have been identified: perindoprilat, perindopril glucuronide, perindoprilat glucuronide, a perindopril lactam, and two perindoprilat lactams. Only perindoprilat is pharmacologically active. Peridoprilat and perindoprilat glucuronide are the two main circulating metabolites.
Perindopril has known human metabolites that include Perindoprilat glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Perindopril, 1.2 hours; Peridoprilat, 30-120 hours. The long half life of peridoprilat is due to its slow dissociation from ACE binding sites.
There are two isoforms of ACE: the somatic isoform, which exists as a glycoprotein comprised of a single polypeptide chain of 1277; and the testicular isoform, which has a lower molecular mass and is thought to play a role in sperm maturation and binding of sperm to the oviduct epithelium. Somatic ACE has two functionally active domains, N and C, which arise from tandem gene duplication. Although the two domains have high sequence similarity, they play distinct physiological roles. The C-domain is predominantly involved in blood pressure regulation while the N-domain plays a role in hematopoietic stem cell differentiation and proliferation. ACE inhibitors bind to and inhibit the activity of both domains, but have much greater affinity for and inhibitory activity against the C-domain. Perindoprilat, the active metabolite of perindopril, competes with ATI for binding to ACE and inhibits and enzymatic proteolysis of ATI to ATII. Decreasing ATII levels in the body decreases blood pressure by inhibiting the pressor effects of ATII as described in the Pharmacology section above. Perindopril also causes an increase in plasma renin activity likely due to a loss of feedback inhibition mediated by ATII on the release of renin and/or stimulation of reflex mechanisms via baroreceptors.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
18
PharmaCompass offers a list of Perindopril API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Perindopril manufacturer or Perindopril supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Perindopril manufacturer or Perindopril supplier.
PharmaCompass also assists you with knowing the Perindopril API Price utilized in the formulation of products. Perindopril API Price is not always fixed or binding as the Perindopril Price is obtained through a variety of data sources. The Perindopril Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Perindopril manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Perindopril, including repackagers and relabelers. The FDA regulates Perindopril manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Perindopril API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Perindopril manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Perindopril supplier is an individual or a company that provides Perindopril active pharmaceutical ingredient (API) or Perindopril finished formulations upon request. The Perindopril suppliers may include Perindopril API manufacturers, exporters, distributors and traders.
click here to find a list of Perindopril suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Perindopril Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Perindopril GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Perindopril GMP manufacturer or Perindopril GMP API supplier for your needs.
A Perindopril CoA (Certificate of Analysis) is a formal document that attests to Perindopril's compliance with Perindopril specifications and serves as a tool for batch-level quality control.
Perindopril CoA mostly includes findings from lab analyses of a specific batch. For each Perindopril CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Perindopril may be tested according to a variety of international standards, such as European Pharmacopoeia (Perindopril EP), Perindopril JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Perindopril USP).
