
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Europe

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
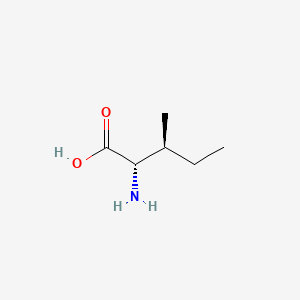

1. Alloisoleucine
2. Isoleucine
3. Isoleucine, L Isomer
4. Isoleucine, L-isomer
5. L-isomer Isoleucine
1. Isoleucine
2. 73-32-5
3. (2s,3s)-2-amino-3-methylpentanoic Acid
4. 2s,3s-isoleucine
5. (s)-isoleucine
6. (s,s)-isoleucine
7. 2-amino-3-methylvaleric Acid
8. L-(+)-isoleucine
9. H-ile-oh
10. Erythro-l-isoleucine
11. L-ile
12. Isoleucine, L-
13. Alpha-amino-beta-methylvaleric Acid
14. Isoleucine (van)
15. Isoleucinum [latin]
16. Isoleucina [spanish]
17. Norvaline, 3-methyl-
18. Valeric Acid, 2-amino-3-methyl-
19. L-norvaline, 3-methyl-, Erythro-
20. Acetic Acid, Amino-sec-butyl-
21. Pentanoic Acid, 2-amino-3-methyl-
22. Ccris 5229
23. Iso-leucine
24. Ile
25. (2s,3s)-alpha-amino-beta-methyl-n-valeric Acid
26. Nsc 46708
27. (2s,3s)-alpha-amino-beta-merthylvaleric Acid
28. (s-(r*,r*))-2-amino-3-methylpentanoic Acid
29. Fema No. 3295
30. (2s,3s)-alpha-amino-beta-merthyl-n-valeric Acid
31. 2-amino-3-methylpentanoic Acid, (s-(r*,r*))-
32. Acetic Acid, Amino(1-methylpropyl)-, (r*,r*)-
33. (2s,3s)-2-amino-3-methylpentanoicacid
34. Isoleucine (l-isoleucine)
35. 5hx0byt4e3
36. Pentanoic Acid, 2-amino-3-methyl-, (s-(r*,r))-
37. Fema No. 4675
38. Chebi:17191
39. [s-(r*,r*)]-2-amino-3-methylpentanoic Acid
40. 2s-amino-3s-methylpentanoic Acid
41. Isoleucine, Dl-
42. Dl-allo-isoleucine
43. Nsc-46708
44. 04y7590d77
45. Mfcd00064222
46. (2s,3s)-alpha-amino-beta-methylvaleric Acid
47. Pentanoic Acid, 2-amino-3-methyl-, (2s,3s)-
48. Isoleucina
49. Isoleucinum
50. Isoleucine [usan:inn]
51. Mfcd00004268
52. H-lle-oh
53. (2s,3s)-2-amino-3-methylpentanoate
54. Einecs 200-798-2
55. Unii-5hx0byt4e3
56. Nsc-9958
57. Nsc46708
58. Laevo-isoleucine
59. (2s,3s)-2-amino-3-methyl-pentanoic Acid
60. L-iso-leucine
61. Hsdb 7798
62. (l)-isoleucine
63. L- Iso-leucine
64. Isoleucine [usan:usp:inn:ban]
65. Unii-04y7590d77
66. Isoleucine (usp)
67. Nsc 9958
68. Einecs 207-139-8
69. H-ile
70. Ile-oh
71. L-isoleucine,(s)
72. (+)-l-isoleucine
73. L-[14c]isoleucine
74. (2s,3s)-a-amino-b-methylvaleric Acid
75. (+/-)-erythro-2-amino-3-methylpentanoic Acid
76. L-isoleucine, 99%
77. Isoleucine [ii]
78. Isoleucine [mi]
79. (2s,3s)-a-amino-b-methyl-n-valeric Acid
80. Ai3-18474
81. L-isoleucine (jp17)
82. Isoleucine [inn]
83. Isoleucine [hsdb]
84. Isoleucine [inci]
85. Isoleucine [usan]
86. Isoleucine Dl-form
87. 2-amino-3-methylvalerate
88. Isoleucine [vandf]
89. Isoleucine, L- (8ci)
90. Bmse000041
91. Bmse000866
92. Bmse000884
93. 2-amino-3-methylpentanoate
94. Isoleucine [mart.]
95. L-isoleucine (h-lle-oh)
96. L-isoleucine [fcc]
97. L-isoleucine [jan]
98. Schembl8869
99. Dl-isoleucine [fcc]
100. H-ile-2-chlorotrityl Resin
101. Isoleucine [who-dd]
102. Sec-c4h9ch(nh2)cooh
103. Acetic Acid, Amino-s-butyl-
104. Dl-isoleucine [fhfi]
105. L-isoleucine (h-l-ile-oh)
106. L-isoleucine [usp-rs]
107. Gtpl3311
108. Chembl1233584
109. Dtxsid1047441
110. Dtxsid2046882
111. Isoleucine Dl-form [mi]
112. L-isoleucine: D-allo-isoleucine
113. Bdbm18140
114. Norvaline, 3-methyl-, Erythro-
115. Isoleucine [ep Monograph]
116. Isoleucine [usp Monograph]
117. L-isoleucine, 99%, Fcc, Fg
118. Pharmakon1600-01301004
119. (2s,3s)-a-amino-b-methylvalerate
120. Hy-n0771
121. Zinc3581355
122. Lmfa01100047
123. Nsc760109
124. S3752
125. L-isoleucine, Vetec(tm), 98.5%
126. Akos015842027
127. (2s,3s)-a-amino-b-methyl-n-valerate
128. Am81842
129. Ccg-266114
130. Cs-w018502
131. Db00167
132. Fd20022
133. Nsc-760109
134. (2s,3s)-2-amino-3-methyl-pentanoate
135. Valine Impurity B [ep Impurity]
136. (2s,3s)-alph-amino-beta-methylvalerate
137. Leucine Impurity A [ep Impurity]
138. (2s,3s)-alpha-amino-beta-methylvalerate
139. Ac-34995
140. As-11616
141. Bp-20357
142. (2s,3s)-alpha-amino-beta-merthylvalerate
143. 064l222
144. [s-(r*,r*)]-2-amino-3-methylpentanoate
145. L-isoleucine, Bioultra, >=99.5% (nt)
146. (2s,3s)-alph-amino-beta-methylvaleric Acid
147. (2s,3s)-alpha-amino-beta-methyl-n-valerate
148. I0181
149. (2s,3s)-alpha-amino-beta-merthyl-n-valerate
150. L-isoleucine, Saj Special Grade, >=99.0%
151. C00407
152. D00065
153. L-isoleucine, Reagent Grade, >=98% (hplc)
154. M03002
155. L-isoleucine, Vetec(tm) Reagent Grade, >=98%
156. L-isoleucine, Cell Culture Reagent (h-l-ile-oh)
157. Q484940
158. Q-201311
159. (2s,3s)-.alpha.-amino-.beta.-methyl-n-valeric Acid
160. .alpha.-amino-.beta.-methylvaleric Acid, (2s,3s)-
161. Pentanoic Acid, 2-amino-3-methyl-, [s-(r*,r*)]-
162. F8880-9085
163. Z1250208653
164. Isoleucine, European Pharmacopoeia (ep) Reference Standard
165. L-isoleucine, Certified Reference Material, Tracecert(r)
166. E46116a2-987c-4709-9e80-a64da838d5a1
167. L-isoleucine, United States Pharmacopeia (usp) Reference Standard
168. L-isoleucine, Pharmaceutical Secondary Standard; Certified Reference Material
169. (s,s)-2-amino-3-methyl-pentanoicacid;(s,s)-isoleucine;[s-(r*,r*)]-2-amino-3-methylpentanoic Acid
170. 1160211-67-5
171. L-isoleucine, From Non-animal Source, Meets Ep, Jp, Usp Testing Specifications, Suitable For Cell Culture, 98.5-101.0%
172. L-isoleucine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 131.17 g/mol |
|---|---|
| Molecular Formula | C6H13NO2 |
| XLogP3 | -1.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 131.094628657 g/mol |
| Monoisotopic Mass | 131.094628657 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 103 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Branched chain amino acid (BCAA)-enriched protein or amino acid mixtures and, in some cases, BCAA alone, have been used in the treatment of a variety of metabolic disorders. These amino acids have received considerable attention in efforts to reduce brain uptake of aromatic amino acids and to raise low circulating levels of BCAA in patients with chronic liver disease and encephalopathy. They have also been used in parenteral nutrition of patients with sepsis and other abnormalities.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 705, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
/Experimental Therapy/ Upper gastrointestinal (GI) bleeding in cirrhotic patients has a high incidence of mortality and morbidity. Postbleeding catabolism has been hypothesized to be partly due to the low biological value of hemoglobin, which lacks the essential amino acid isoleucine. The aims were to study the metabolic consequences of a "simulated" upper GI bleed in patients with cirrhosis of the liver and the effects of intravenous infusion of isoleucine. Portal drained viscera, liver, muscle, and kidney protein kinetics were quantified using a multicatheterization technique during routine portography. Sixteen overnight-fasted, metabolically stable patients who received an intragastric infusion of an amino acid solution mimicking hemoglobin every 4 hours were randomized to saline or isoleucine infusion and received a mixture of stable isotopes (L-[ring-2H5]phenylalanine, L-[ring-2H4]tyrosine, and L-[ring-2H2]tyrosine) to determine organ protein kinetics. This simulated bleed resulted in hypoisoleucinemia that was attenuated by isoleucine infusion. Isoleucine infusion during the bleed resulted in a positive net balance of phenylalanine across liver and muscle, whereas renal and portal drained viscera protein kinetics were unaffected. In the control group, no significant effect was shown...
Olde Damink SWM et al; Hepatology 45 (3): 560-8 (2007). Available from, as of March 17, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17326149
The branched-chain amino acids may have antihepatic encephalopathy activity in some. They may also have anticatabolic and antitardive dyskinesia activity.
They provide ingredients for the manufacturing of other essential biochemical components in the body, some of which are utilized for the production of energy, stimulants to the upper brain and helping you to be more alert.
Absorption
Absorbed from the small intestine by a sodium-dependent active-transport process
Although the free amino acids dissolved in the body fluids are only a very small proportion of the body's total mass of amino acids, they are very important for the nutritional and metabolic control of the body's proteins. ... Although the plasma compartment is most easily sampled, the concentration of most amino acids is higher in tissue intracellular pools. Typically, large neutral amino acids, such as leucine and phenylalanine, are essentially in equilibrium with the plasma. Others, notably glutamine, glutamic acid, and glycine, are 10- to 50-fold more concentrated in the intracellular pool. Dietary variations or pathological conditions can result in substantial changes in the concentrations of the individual free amino acids in both the plasma and tissue pools. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 596, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
After ingestion, proteins are denatured by the acid in the stomach, where they are also cleaved into smaller peptides by the enzyme pepsin, which is activated by the increase in stomach acidity that occurs on feeding. The proteins and peptides then pass into the small intestine, where the peptide bonds are hydrolyzed by a variety of enzymes. These bond-specific enzymes originate in the pancreas and include trypsin, chymotrypsins, elastase, and carboxypeptidases. The resultant mixture of free amino acids and small peptides is then transported into the mucosal cells by a number of carrier systems for specific amino acids and for di- and tri-peptides, each specific for a limited range of peptide substrates. After intracellular hydrolysis of the absorbed peptides, the free amino acids are then secreted into the portal blood by other specific carrier systems in the mucosal cell or are further metabolized within the cell itself. Absorbed amino acids pass into the liver, where a portion of the amino acids are taken up and used; the remainder pass through into the systemic circulation and are utilized by the peripheral tissues. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 599, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The intraerythrocytic malaria parasite derives much of its requirement for amino acids from the digestion of the hemoglobin of its host cell. However, one amino acid, isoleucine, is absent from adult human hemoglobin and must therefore be obtained from the extracellular medium. ... The mechanisms involved in the uptake of isoleucine by the intraerythrocytic parasite /are characterized/. Under physiologic conditions the rate of transport of isoleucine into human erythrocytes infected with mature trophozoite-stage Plasmodium falciparum parasites is increased to approximately 5-fold that in uninfected cells, with the increased flux being via the new permeability pathways (NPPs) induced by the parasite in the host cell membrane. Transport via the NPPs ensures that protein synthesis is not rate limited by the flux of isoleucine across the erythrocyte membrane. On entering the infected erythrocyte, isoleucine is taken up into the parasite via a saturable, ATP-, Na+-, and H+-independent system which has the capacity to mediate the influx of isoleucine in exchange for leucine (liberated from hemoglobin). The accumulation of radiolabeled isoleucine within the parasite is mediated by a second (high-affinity, ATP-dependent) mechanism, perhaps involving metabolism and/or the concentration of isoleucine within an intracellular organelle.
Martin RE, Kirk K; Blood 109 (5): 2217-24 (2007). Available from, as of March 17, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17047158
Hepatic
The branched-chain amino acids (BCAA) -- leucine, isoleucine, and valine -- differ from most other indispensable amino acids in that the enzymes initially responsible for their catabolism are found primarily in extrahepatic tissues. Each undergoes reversible transamination, catalyzed by a branched-chain aminotransferase (BCAT), and yields alpha-ketoisocaproate (KIC, from leucine), alpha-keto-beta-methylvalerate (KMV, from isoleucine), and alpha-ketoisovalerate (KIV, from valine). Each of these ketoacids then undergoes an irreversible, oxidative decarboxylation, catalyzed by a branchedchain ketoacid dehydrogenase (BCKAD). The latter is a multienzyme system located in mitochondrial membranes. The products of these oxidation reactions undergo further transformations to yield acetyl CoA, propionyl CoA, acetoacetate, and succinyl CoA; the BCAA are thus keto- and glucogenic.
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 704, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Once the amino acid deamination products enter the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Krebs cycle) or the glycolytic pathway, their carbon skeletons are also available for use in biosynthetic pathways, particularly for glucose and fat. Whether glucose or fat is formed from the carbon skeleton of an amino acid depends on its point of entry into these two pathways. If they enter as acetyl-CoA, then only fat or ketone bodies can be formed. The carbon skeletons of other amino acids can, however, enter the pathways in such a way that their carbons can be used for gluconeogenesis. This is the basis for the classical nutritional description of amino acids as either ketogenic or glucogenic (ie, able to give rise to either ketones [or fat] or glucose). Some amino acids produce both products upon degradation and so are considered both ketogenic and glucogenic. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 606, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Mutations in the HSD17B10 gene were identified in two previously described mentally retarded males. A point mutation c.776G>C was found from a survivor (SV), whereas a potent mutation, c.419C>T, was identified in another deceased case (SF) with undetectable hydroxysteroid (17beta) dehydrogenase 10 (HSD10) activity. Protein levels of mutant HSD10(R130C) in patient SF and HSD10(E249Q) in patient SV were about half that of HSD10 in normal controls. The E249Q mutation appears to affect HSD10 subunit interactions, resulting in an allosteric regulatory enzyme. For catalyzing the oxidation of allopregnanolone by NAD+ the Hill coefficient of the mutant enzyme is approximately 1.3. HSD10(E249Q) was unable to catalyze the dehydrogenation of 2-methyl-3-hydroxybutyryl-CoA and the oxidation of allopregnanolone, a positive modulator of the gamma-aminobutyric acid type A receptor, at low substrate concentrations. Neurosteroid homeostasis is critical for normal cognitive development, and there is increasing evidence that a blockade of isoleucine catabolism alone does not commonly cause developmental disabilities. The results support the theory that an imbalance in neurosteroid metabolism could be a major cause of the neurological handicap associated with hydroxysteroid (17beta) dehydrogenase 10 deficiency.
Yang S-Y et al; Proc Nat Acad Sci 106 (35): 14820-4 (2009). Available from, as of March 17, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=19706438
The HSD17B10 gene maps on chromosome Xp11.2, a region highly associated with X-linked mental retardation. This gene encodes HSD10, a mitochondrial multifunctional enzyme that plays a significant part in the metabolism of neuroactive steroids and the degradation of isoleucine. The HSD17B10 gene is composed of six exons and five introns. Its exon 5 is an alternative exon such that there are several HSD17B10 mRNA isoforms in brain. A silent mutation (c.605C-->A) and three missense mutations (c.395C-->G; c.419C-->T; c.771A-->G), respectively, cause the X-linked mental retardation, choreoathetosis, and abnormal behavior (MRXS10) and the hydroxyacyl-CoA dehydrogenase II deficiency...
Yang S-Y et al; Mol Genetics Metab 92 (102): 36-42 (2007). Available from, as of March 17, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17618155
Human type 10 17beta-hydroxysteroid dehydrogenase (HSD) is a homotetrameric protein located in mitochondria. This enzyme was alternatively named short chain L-3-hydroxyacyl-CoA dehydrogenase (SCHSD). This NAD(H)-dependent dehydrogenase is essential for the metabolism of branched-chain fatty acids and isoleucine ...
He X-Y, Yang S-Y; Endocrine Metabol Immune Disorders Drug Targets 6 (1): 95-102 (2006). Available from, as of March 17, 2010: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=16611167
(Applies to Valine, Leucine and Isoleucine)
This group of essential amino acids are identified as the branched-chain amino acids, BCAAs. Because this arrangement of carbon atoms cannot be made by humans, these amino acids are an essential element in the diet. The catabolism of all three compounds initiates in muscle and yields NADH and FADH2 which can be utilized for ATP generation. The catabolism of all three of these amino acids uses the same enzymes in the first two steps. The first step in each case is a transamination using a single BCAA aminotransferase, with a-ketoglutarate as amine acceptor. As a result, three different a-keto acids are produced and are oxidized using a common branched-chain a-keto acid dehydrogenase, yielding the three different CoA derivatives. Subsequently the metabolic pathways diverge, producing many intermediates.
The principal product from valine is propionylCoA, the glucogenic precursor of succinyl-CoA. Isoleucine catabolism terminates with production of acetylCoA and propionylCoA; thus isoleucine is both glucogenic and ketogenic. Leucine gives rise to acetylCoA and acetoacetylCoA, and is thus classified as strictly ketogenic.
There are a number of genetic diseases associated with faulty catabolism of the BCAAs. The most common defect is in the branched-chain a-keto acid dehydrogenase. Since there is only one dehydrogenase enzyme for all three amino acids, all three a-keto acids accumulate and are excreted in the urine. The disease is known as Maple syrup urine disease because of the characteristic odor of the urine in afflicted individuals. Mental retardation in these cases is extensive. Unfortunately, since these are essential amino acids, they cannot be heavily restricted in the diet; ultimately, the life of afflicted individuals is short and development is abnormal The main neurological problems are due to poor formation of myelin in the CNS.
Amino acids are selected for protein synthesis by binding with transfer RNA (tRNA) in the cell cytoplasm. The information on the amino acid sequence of each individual protein is contained in the sequence of nucleotides in the messenger RNA (mRNA) molecules, which are synthesized in the nucleus from regions of DNA by the process of transcription. The mRNA molecules then interact with various tRNA molecules attached to specific amino acids in the cytoplasm to synthesize the specific protein by linking together individual amino acids; this process, known as translation, is regulated by amino acids (e.g., leucine), and hormones. Which specific proteins are expressed in any particular cell and the relative rates at which the different cellular proteins are synthesized, are determined by the relative abundances of the different mRNAs and the availability of specific tRNA-amino acid combinations, and hence by the rate of transcription and the stability of the messages. From a nutritional and metabolic point of view, it is important to recognize that protein synthesis is a continuing process that takes place in most cells of the body. In a steady state, when neither net growth nor protein loss is occurring, protein synthesis is balanced by an equal amount of protein degradation. The major consequence of inadequate protein intakes, or diets low or lacking in specific indispensable amino acids relative to other amino acids (often termed limiting amino acids), is a shift in this balance so that rates of synthesis of some body proteins decrease while protein degradation continues, thus providing an endogenous source of those amino acids most in need. /Protein synthesis/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 601-602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
The mechanism of intracellular protein degradation, by which protein is hydrolyzed to free amino acids, is more complex and is not as well characterized at the mechanistic level as that of synthesis. A wide variety of different enzymes that are capable of splitting peptide bonds are present in cells. However, the bulk of cellular proteolysis seems to be shared between two multienzyme systems: the lysosomal and proteasomal systems. The lysosome is a membrane-enclosed vesicle inside the cell that contains a variety of proteolytic enzymes and operates mostly at acid pH. Volumes of the cytoplasm are engulfed (autophagy) and are then subjected to the action of the protease enzymes at high concentration. This system is thought to be relatively unselective in most cases, although it can also degrade specific intracellular proteins. The system is highly regulated by hormones such as insulin and glucocorticoids, and by amino acids. The second system is the ATP-dependent ubiquitin-proteasome system, which is present in the cytoplasm. The first step is to join molecules of ubiquitin, a basic 76-amino acid peptide, to lysine residues in the target protein. Several enzymes are involved in this process, which selectively targets proteins for degradation by a second component, the proteasome. /Amino acids/
NAS, Food and Nutrition Board, Institute of Medicine; Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients). National Academy Press, Washington, D.C., pg. 602, 2009. Available from, as of March 10, 2010: https://www.nap.edu/catalog/10490.html
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
75
PharmaCompass offers a list of Isoleucine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Isoleucine manufacturer or Isoleucine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Isoleucine manufacturer or Isoleucine supplier.
PharmaCompass also assists you with knowing the Isoleucine API Price utilized in the formulation of products. Isoleucine API Price is not always fixed or binding as the Isoleucine Price is obtained through a variety of data sources. The Isoleucine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Isoleucine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Isoleucine, including repackagers and relabelers. The FDA regulates Isoleucine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Isoleucine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Isoleucine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Isoleucine supplier is an individual or a company that provides Isoleucine active pharmaceutical ingredient (API) or Isoleucine finished formulations upon request. The Isoleucine suppliers may include Isoleucine API manufacturers, exporters, distributors and traders.
click here to find a list of Isoleucine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Isoleucine DMF (Drug Master File) is a document detailing the whole manufacturing process of Isoleucine active pharmaceutical ingredient (API) in detail. Different forms of Isoleucine DMFs exist exist since differing nations have different regulations, such as Isoleucine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Isoleucine DMF submitted to regulatory agencies in the US is known as a USDMF. Isoleucine USDMF includes data on Isoleucine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Isoleucine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Isoleucine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Isoleucine Drug Master File in Japan (Isoleucine JDMF) empowers Isoleucine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Isoleucine JDMF during the approval evaluation for pharmaceutical products. At the time of Isoleucine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Isoleucine suppliers with JDMF on PharmaCompass.
A Isoleucine CEP of the European Pharmacopoeia monograph is often referred to as a Isoleucine Certificate of Suitability (COS). The purpose of a Isoleucine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Isoleucine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Isoleucine to their clients by showing that a Isoleucine CEP has been issued for it. The manufacturer submits a Isoleucine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Isoleucine CEP holder for the record. Additionally, the data presented in the Isoleucine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Isoleucine DMF.
A Isoleucine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Isoleucine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Isoleucine suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Isoleucine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Isoleucine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Isoleucine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Isoleucine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Isoleucine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Isoleucine suppliers with NDC on PharmaCompass.
Isoleucine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Isoleucine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Isoleucine GMP manufacturer or Isoleucine GMP API supplier for your needs.
A Isoleucine CoA (Certificate of Analysis) is a formal document that attests to Isoleucine's compliance with Isoleucine specifications and serves as a tool for batch-level quality control.
Isoleucine CoA mostly includes findings from lab analyses of a specific batch. For each Isoleucine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Isoleucine may be tested according to a variety of international standards, such as European Pharmacopoeia (Isoleucine EP), Isoleucine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Isoleucine USP).
