
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aristada
1. 1259305-29-7
2. Aristada
3. Rdc-3317
4. Alks 9072
5. Aristada Initio
6. Aripiprazole Lauroxil [usan]
7. Alks 9070
8. Rdc 3317
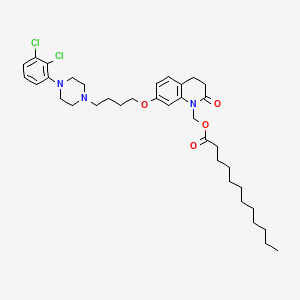
9. [7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-2-oxo-3,4-dihydroquinolin-1-yl]methyl Dodecanoate
10. Alks-9070
11. Alks-9072
12. Rdc3317
13. B786j7a343
14. Aripiprazole Lauroxil (usan)
15. Dodecanoic Acid, (7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-2-oxo-1(2h)-quinolinyl)methyl Ester
16. (7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-oxo-3,4-dihydroquinolin-1(2h)-yl)methyl Dodecanoate
17. Unii-b786j7a343
18. Aristada (tn)
19. [7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-2-oxo-3,4-dihydroquinolin-1(2h)-yl]methyl Dodecanoate
20. Aripiprazole-lauroxil
21. Aripiprazol- Lauroxil
22. Schembl1044330
23. Chembl2219425
24. Chebi:90930
25. Dtxsid60154997
26. Aripiprazole Lauroxil [mi]
27. Bcp14993
28. Ex-a4069
29. Mfcd26967976
30. Zinc95564895
31. Aripiprazole Lauroxil [who-dd]
32. Db14185
33. Ncgc00532501-01
34. Ac-36504
35. As-35241
36. Aripiprazole Lauroxil [orange Book]
37. Hy-108751
38. Cs-0030962
39. D10364
40. A856290
41. Q25323757
42. Dodecanoic Acid [7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2-oxo-1(2h)-quinolinyl]methyl Ester
| Molecular Weight | 660.7 g/mol |
|---|---|
| Molecular Formula | C36H51Cl2N3O4 |
| XLogP3 | 10 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 20 |
| Exact Mass | 659.3256625 g/mol |
| Monoisotopic Mass | 659.3256625 g/mol |
| Topological Polar Surface Area | 62.3 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 858 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Aripiprazole lauroxil is indicated for the treatment of schizophrenia and related psychotic disorders.
FDA Label
Aripiprazole, which is a major pharmacological metabolite of aripiprazole lauroxil, serves to improve the positive and negative symptoms of schizophrenia by modulating dopaminergic signalling pathways. Aripiprazole lauroxil is reported to have minimal effects on sexual function or prolactin levels.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Absorption
Following a single extended-release intramuscular injection of aripiprazole lauroxil, aripiprazole can be detected in the systemic circulation from 5 to 6 days and is continued to be released for an additional 36 days. The concentrations of aripiprazole increases with consecutive doses of aripiprazole lauroxil and the steady state is reached following the fourth monthly injection. The systemic exposure to aripiprazole was similar when comparing deltoid and gluteal intramuscular injections.
Route of Elimination
Based on the pharmacokinetic study for aripiprazole, less than 1% of unchanged aripiprazole was excreted in the urine and approximately 18% of the oral dose was recovered unchanged in the feces.
Volume of Distribution
Based on population pharmacokinetic analysis, the apparent volume of distribution of aripiprazole following intramuscular injection of aripiprazole lauroxil was 268 L, indicating extensive extravascular distribution following absorption. Health human volunteer study indicates that aripiprazole crosses the blood-brain barrier.
Clearance
In rats, the clearance for aripiprazole lauroxil was 0.32 0.11 L/h/kg following injection of aripiprazole lauroxil molar equivalent to 5 mg aripiprazole/kg.
Aripiprazole lauroxil is hydrolyzed to form N-hydroxymethyl-aripiprazole via esterases. N-hydroxymethyl-aripiprazole undergoes a rapid, nonenzymatic spontaneous cleavage, or water-mediated hydrolysis, to form aripiprazole, which mainly contributes to the pharmacological actions of aripiprazole lauroxil. Aripiprazole is further metabolized by hepatic CYP3A4 and CYP2D6 to form dehydro-aripiprazole, which retains some pharmacological activity. Dehydro-aripiprazole displays affinities for D2 receptors similar to aripiprazole and represents 30-40% of the aripiprazole exposure in plasma. Cytochrome P450 2D6 is subject to genetic polymorphism, which results in pharmacokinetic differences among CYP2D6 metabolizer phenotypes and dosage adjustments accordingly.
The mean aripiprazole terminal elimination half-life ranged from 29.2 days to 34.9 days after every 4-week injection of aripiprazole lauroxil 441, 662 and 882 mg.
The pharmacological activity of aripiprazole lauroxil is thought to be mainly mediated by its metabolite aripiprazole, and to a lesser extent, dehydro-aripiprazole. Aripiprazole functions as a partial agonist at the dopamine D2 and the serotonin 5-HT1A receptors, and as an antagonist at the serotonin 5-HT2A receptor. The desired outcome of antipsuchotic agents in schizophrenia is to inhibit dopaminergic transmission in the limbic system and enhance dopaminergic transmission in the prefrontal cortex. As a partial agonist at D2 receptors in the mesolimbic dopaminergic pathway, aripiprazole acts as a functional antagonist in the mesolimbic dopamine pathway and reduces the extent of dopaminergic pathway activity. This results in reduced positive symptoms in schizophrenia and extrapyramidal motor side effects. In contrast, aripiprazole is thought to act as a functional agonist in the mesocortical pathway, where reduced dopamine activity is seen in association with negative symptoms and cognitive impairment. Antagonism at 5-HT2A receptors by aripiprazole alleviates the negative symptoms and cognitive impairment of schizophrenia. 5-HT2A receptors are Gi/Go-coupled that upon activation, produce neuronal inhibition via decreased neuronal excitability and decreased transmitter release at the nerve terminals. In the nigrostriatal pathway, 5-HT2A regulates the release of dopamine. Through antagonism of 5-HT2A receptors, aripiprazole disinhibits the release of dopamine in the striatum and enhance the levels of the transmitters at the nerve terminals. The combined effects of D2 and 5-HT2A antagonism are thought to counteract the increased dopamine function causing increased extrapyramidal side effects. Blocking 5-HT2A receptors may also lead to the modulation of glutamate release in the mesocortical circuit, which is a transmitter that plays a role in schizophrenia. 5-HT1A receptors are autoreceptors that inhibit 5-HT release upon activation. Aripiprazole is a partial agonist at theses receptors and reduces 5-HT release; this results in potentiated dopamine release in the striatum and prefrontal cortex. It is reported that therapeutic doses of aripiprazole occupies up to 90% of brain D2 receptors in a dose-dependent manner. Apripiprazole targets different receptors that lead to drug-related adverse reactions; for example, the antagonist activity at the alpha-1 adrenergic receptors results in orthostatic hypotension. Aripiprazole's antagonism of histamine H1 receptors may explain the somnolence observed with this drug.
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39954
Submission : 2024-07-30
Status : Active
Type : II

NDC Package Code : 64552-4079
Start Marketing Date : 2015-10-05
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32683
Submission : 2018-03-31
Status : Active
Type : II

NDC Package Code : 58032-1020
Start Marketing Date : 2018-10-25
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF |

 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.

NDC Package Code : 69037-0045
Start Marketing Date : 2015-10-05
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32147
Submission : 2017-10-31
Status : Active
Type : II

NDC Package Code : 50370-0055
Start Marketing Date : 2017-07-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : Reviewed
Rev. Date : 2022-03-31
Pay. Date : 2021-12-14
DMF Number : 36522
Submission : 2021-12-22
Status : Active
Type : II



NDC Package Code : 59116-5070
Start Marketing Date : 2017-09-19
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Abilify (aripiprazole) action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and for bipolar I disorder in adults.
Lead Product(s): Aripiprazole,Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Asimtufii
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Otsuka Holdings
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole,Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Otsuka Holdings
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Approves Otsuka and Lundbeck’s ABILIFY ASIMTUFII® (aripiprazole), the First Once-Every-Two-...
Details : Abilify (aripiprazole) action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and for bipolar I disorder in adults.
Product Name : Abilify Asimtufii
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 28, 2023

Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Alkermes Plc
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 22, 2022

Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Alkermes Plc
Deal Size : Inapplicable
Deal Type : Inapplicable
C-Cog in Early Course Schizophrenia Study
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The objective of the randomized, double-blind, placebo-controlled, multicenter study is to determine if high-dose Octagam® 10% therapy will slow or stop respiratory deterioration in patients with severe coronavirus disease.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Infections and Infectious Diseases Brand Name: Aristada Initio
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 20, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Approves Octapharma USA Investigational New Drug Application for Severe COVID-19 Patients
Details : The objective of the randomized, double-blind, placebo-controlled, multicenter study is to determine if high-dose Octagam® 10% therapy will slow or stop respiratory deterioration in patients with severe coronavirus disease.
Product Name : Aristada Initio
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 20, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Results from the study provide evidence to support the use of the ARISTADA INITIO one-day regimen together with ARISTADA for treatment of an acute exacerbation of schizophrenia, started in the hospital setting and continued through the critical transition to outpatient care.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 20, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Results from the study provide evidence to support the use of the ARISTADA INITIO one-day regimen together with ARISTADA for treatment of an acute exacerbation of schizophrenia, started in the hospital setting and continued through the critical transitio...
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 20, 2020

Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Al-Ncd
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Alkermes Plc
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 18, 2019

Lead Product(s) : Aripiprazole Lauroxil,Al-Ncd
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Alkermes Plc
Deal Size : Inapplicable
Deal Type : Inapplicable
Aripiprazole Lauroxil for Preventing Psychotic Relapse After an Initial Schizophrenia Episode
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 18, 2019

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 17, 2017

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of Aripiprazole Lauroxil or Paliperidone Palmitate for the Treatment of Schizophrenia
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 17, 2017

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 22, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of Aripiprazole Lauroxil in Subjects With Schizophrenia or Schizoaffective Disorder
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 22, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 18, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of Aripiprazole Lauroxil (Also Known as ARISTADA TM) in Subjects With Schizophrenia
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase IV clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 18, 2015

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Schizophrenia.
Lead Product(s): Aripiprazole Lauroxil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 19, 2014

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole Lauroxil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
An Open-Label Study of Aripiprazole Lauroxil in Subjects With Stable Schizophrenia
Details : Aripiprazole Lauroxil is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Schizophrenia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 19, 2014

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info : USA
Registration Country : Greece
Brand Name :
Dosage Form : Long Acting Injectable
Dosage Strength : 441MG/SYRINGE
Packaging :
Approval Date :
Application Number :
Regulatory Info : USA
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info : USA
Registration Country : Greece
Brand Name :
Dosage Form : Long Acting Injectable
Dosage Strength : 662MG/SYRINGE
Packaging :
Approval Date :
Application Number :
Regulatory Info : USA
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info : USA
Registration Country : Greece
Brand Name :
Dosage Form : Long Acting Injectable
Dosage Strength : 882MG/SYRINGE
Packaging :
Approval Date :
Application Number :
Regulatory Info : USA
Registration Country : Greece
 Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Pharmathen provides life cycle Management solutions for branded pharma, as well as to develop & establish new technology platforms.
Regulatory Info : USA
Registration Country : Greece
Brand Name :
Dosage Form : Long Acting Injectable
Dosage Strength : 1064MG/SYRINGE
Packaging :
Approval Date :
Application Number :
Regulatory Info : USA
Registration Country : Greece
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARISTADA
Dosage Form : SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 441MG/1.6ML (275.63MG/ML)
Packaging :
Approval Date : 2015-10-05
Application Number : 207533
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARISTADA
Dosage Form : SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 662MG/2.4ML (275.83MG/ML)
Packaging :
Approval Date : 2015-10-05
Application Number : 207533
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARISTADA
Dosage Form : SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 882MG/3.2ML (275.63MG/ML)
Packaging :
Approval Date : 2015-10-05
Application Number : 207533
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARISTADA
Dosage Form : SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 1064MG/3.9ML (272.82MG/ML)
Packaging :
Approval Date : 2017-06-05
Application Number : 207533
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARISTADA INITIO KIT
Dosage Form : SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 675MG/2.4ML (281.25MG/ML)
Packaging :
Approval Date : 2018-06-29
Application Number : 209830
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Company : Alkermes Inc
Aripiprazole Lauroxil
Drug Cost (USD) : 272,878,291
Year : 2023
Prescribers : 13295
Prescriptions : 90583

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil,Submicr.
Drug Cost (USD) : 3,295,869
Year : 2023
Prescribers : 1249
Prescriptions : 1362

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil
Drug Cost (USD) : 256,918,832
Year : 2022
Prescribers : 13161
Prescriptions : 88507

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil,Submicr.
Drug Cost (USD) : 2,947,809
Year : 2022
Prescribers : 1173
Prescriptions : 1273

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil
Drug Cost (USD) : 230,380,653
Year : 2021
Prescribers : 12813
Prescriptions : 82292

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil,Submicr.
Drug Cost (USD) : 3,236,337
Year : 2021
Prescribers : 1332
Prescriptions : 1443

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil
Drug Cost (USD) : 203,135,527
Year : 2020
Prescribers : 11836
Prescriptions : 76175

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil,Submicr.
Drug Cost (USD) : 2,491,372
Year : 2020
Prescribers : 1067
Prescriptions : 1163

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil
Drug Cost (USD) : 160,454,398
Year : 2019
Prescribers : 10998
Prescriptions : 64816

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Alkermes Inc
Aripiprazole Lauroxil,Submicr.
Drug Cost (USD) : 2,246,756
Year : 2019
Prescribers : 1056
Prescriptions : 1106

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
18 Jun 2022
Reply
20 Dec 2016
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2039-04-06
US Patent Number : 11273158
Drug Substance Claim :
Drug Product Claim :
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2039-04-06

Patent Expiration Date : 2032-11-07
US Patent Number : 9034867
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2032-11-07

Patent Expiration Date : 2033-09-19
US Patent Number : 12311027
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2033-09-19

Patent Expiration Date : 2033-09-19
US Patent Number : 12311027
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2033-09-19

Patent Expiration Date : 2035-03-19
US Patent Number : 10813928
Drug Substance Claim :
Drug Product Claim :
Application Number : 207533
Patent Use Code : U-2983
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-03-19

Patent Expiration Date : 2035-03-19
US Patent Number : 10813928
Drug Substance Claim :
Drug Product Claim :
Application Number : 207533
Patent Use Code : U-2983
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-03-19

Patent Expiration Date : 2030-06-24
US Patent Number : 10112903
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2030-06-24

Patent Expiration Date : 2030-06-24
US Patent Number : 10112903
Drug Substance Claim : Y
Drug Product Claim :
Application Number : 209830
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2030-06-24

Patent Expiration Date : 2030-06-24
US Patent Number : 8796276
Drug Substance Claim :
Drug Product Claim :
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2030-06-24

Patent Expiration Date : 2033-10-24
US Patent Number : 9193685
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207533
Patent Use Code : U-543
Delist Requested :
Patent Use Description : TREATMENT OF SCHIZOPHR...
Patent Expiration Date : 2033-10-24

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ANALYTICAL

ABOUT THIS PAGE
53
PharmaCompass offers a list of Aripiprazole Lauroxil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aripiprazole Lauroxil manufacturer or Aripiprazole Lauroxil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aripiprazole Lauroxil manufacturer or Aripiprazole Lauroxil supplier.
PharmaCompass also assists you with knowing the Aripiprazole Lauroxil API Price utilized in the formulation of products. Aripiprazole Lauroxil API Price is not always fixed or binding as the Aripiprazole Lauroxil Price is obtained through a variety of data sources. The Aripiprazole Lauroxil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aristada manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aristada, including repackagers and relabelers. The FDA regulates Aristada manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aristada API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aristada manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aristada supplier is an individual or a company that provides Aristada active pharmaceutical ingredient (API) or Aristada finished formulations upon request. The Aristada suppliers may include Aristada API manufacturers, exporters, distributors and traders.
click here to find a list of Aristada suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aristada DMF (Drug Master File) is a document detailing the whole manufacturing process of Aristada active pharmaceutical ingredient (API) in detail. Different forms of Aristada DMFs exist exist since differing nations have different regulations, such as Aristada USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aristada DMF submitted to regulatory agencies in the US is known as a USDMF. Aristada USDMF includes data on Aristada's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aristada USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aristada suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aristada as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aristada API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aristada as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aristada and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aristada NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aristada suppliers with NDC on PharmaCompass.
Aristada Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aristada GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aristada GMP manufacturer or Aristada GMP API supplier for your needs.
A Aristada CoA (Certificate of Analysis) is a formal document that attests to Aristada's compliance with Aristada specifications and serves as a tool for batch-level quality control.
Aristada CoA mostly includes findings from lab analyses of a specific batch. For each Aristada CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aristada may be tested according to a variety of international standards, such as European Pharmacopoeia (Aristada EP), Aristada JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aristada USP).

