
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
Australia

Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
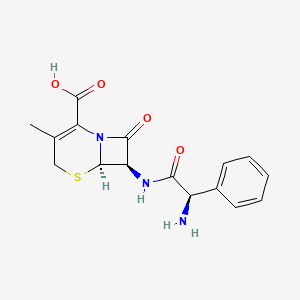

1. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r-(6alpha,7beta(r*)))-
2. Cefalexin
3. Cephalexin Dihydride
4. Cephalexin Hemihydrate
5. Cephalexin Hydrochloride
6. Cephalexin Monohydrate
7. Cephalexin Monohydrochloride
8. Cephalexin Monohydrochloride, Monohydrate
9. Cephalexin, (6r-(6alpha,7alpha(r*)))-isomer
10. Cephalexin, (6r-(6alpha,7beta(s*)))-isomer
11. Cephalexin, (6r-(6alpha,7beta))-isomer
12. Cephalexin, Monosodium Salt
13. Cephalexin, Monosodium Salt, (6r-(6alpha,7beta))-isomer
14. Ceporexine
15. Palitrex
1. Cefalexin
2. 15686-71-2
3. Cephacillin
4. Keflex
5. Ceporexin
6. Cepexin
7. Cephalexinum
8. Cepastar
9. Cephalexine
10. Celexin
11. Ceporex
12. Alcephin
13. Alsporin
14. Carnosporin
15. Cefablan
16. Cefaleksin
17. Cefalexina
18. Cefalexine
19. Cefalexinum
20. Durantel
21. Mamalexin
22. Sinthecillin
23. Uphalexin
24. Cefadin
25. Felexin
26. Pyassan
27. Tepaxin
28. Keflet
29. Palitrex
30. Cefalessina [dcit]
31. Cephalexin Anhydrous
32. Cefaseptin
33. Novolexin
34. Biocef
35. Keftab
36. Cefalexine [inn-french]
37. Cefalexinum [inn-latin]
38. Cefalexina [inn-spanish]
39. Panixine Disperdose
40. Lilly 66873
41. 7-(d-alpha-aminophenylacetamido)desacetoxycephalosporanic Acid
42. Ceporexine
43. Cefalexin Anhydrous
44. Cefalexin [inn]
45. Anhydrous Cephalexin
46. Ceffanex
47. Cephanasten
48. 7-beta-(d-alpha-amino-alpha-phenylacetylamino)-3-methyl-3-cephem-4-carboxylic Acid
49. Cefaloto
50. Ceforal
51. Chebi:3534
52. Cophalexin
53. Factagard
54. Kefalospes
55. Lexibiotico
56. Lopilexin
57. Neolexina
58. Ortisporina
59. Sartosona
60. Sencephalin
61. Servispor
62. Tokiolexin
63. Cefadal
64. Cefadina
65. Cefalin
66. Cefovit
67. Cephaxin
68. Erocetin
69. Ibrexin
70. Inphalex
71. Kefolan
72. Kekrinal
73. Kidolex
74. Lafarine
75. Larixin
76. Lenocef
77. Lonflex
78. Madlexin
79. Mamlexin
80. Medoxine
81. Oriphex
82. Ospexin
83. Pectril
84. Sanaxin
85. Sepexin
86. Sialexin
87. Sporicef
88. Sporidex
89. Zozarine
90. Alexin
91. Cefax
92. Cephin
93. Cepol
94. Check
95. Fexin
96. Ibilex
97. Neokef
98. Nufex
99. Oracef
100. Oroxin
101. Roceph
102. Syncl
103. Syncle
104. Synecl
105. Voxxim
106. Winlex
107. Cex
108. Cefa-iskia
109. Ceporex Forte
110. Ceporexin-e
111. Sq 20248
112. Chembl1727
113. Durantel Ds
114. L-keflex
115. (6r,7r)-7-{[(2r)-2-amino-2-phenylacetyl]amino}-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
116. Cefalessina
117. Ed A-ceph
118. Roceph Distab
119. 5sff1w6677
120. Ncgc00159522-02
121. Ceflax
122. Dsstox_cid_2780
123. Dsstox_rid_76726
124. Dsstox_gsid_22780
125. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
126. (6r,7r)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
127. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 7-[[(2r)-aminophenylacetyl]amino]-3-methyl-8-oxo-, (6r,7r)-
128. L-cephalexin
129. Cephalexin 1-hydrate
130. Cerexin
131. Optocef
132. Cephalexin [usan:ban]
133. (6r,7r)-7-[(2r)-2-amino-2-phenylacetamido]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
134. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2r)-aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r,7r)-
135. Smr000338536
136. S 6437
137. Cas-15686-71-2
138. Keflex (tn)
139. Hsdb 3022
140. Nsc-758162
141. Einecs 239-773-6
142. Brn 0965503
143. Taicelexin
144. 7-(d-2-amino-2-phenylacetamido)-3-methyl-delta3-cephem-4-carboxylic Acid
145. Cerexins
146. Amplex
147. Unii-5sff1w6677
148. Anhydrous Cefalexin
149. Cefalexin,(s)
150. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid
151. 34632-04-7
152. Cefadros
153. Cephamasten
154. Efalexin
155. Garasin
156. Iwalexin
157. Kefloridina
158. Oracocin
159. Cefalexin (jp17)
160. Cephalexin (cefalexin)
161. Cefalexin [jan]
162. Cephalexin [mi]
163. Prestwick0_000358
164. Prestwick1_000358
165. Prestwick2_000358
166. Prestwick3_000358
167. Cephalexin [hsdb]
168. Epitope Id:117132
169. Cefalexin [who-dd]
170. Schembl2961
171. 7-(d-2-amino-2-phenylacetamido)-3-methyl-delta (sup 3)-cephem-4- Carboxylic Acid
172. Bspbio_000455
173. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure
174. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-, (6r-(6alpha,7beta(r*)))-
175. Mls000759527
176. Mls001424036
177. Spbio_002376
178. Bpbio1_000501
179. Gtpl4832
180. Dtxsid9022780
181. Lilly-66873
182. Bcpp000289
183. Hms2051a04
184. Hy-b0200
185. Zinc3830500
186. Tox21_111740
187. Bdbm50139896
188. Akos004119846
189. Tox21_111740_1
190. Bcp9000509
191. Ccg-100831
192. Cs-2137
193. Db00567
194. Nc00081
195. Nsc 758162
196. Ncgc00159522-03
197. Ncgc00159522-05
198. (6r,7r)-7-[[(2r)-2-amino-2-phenyl-acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
199. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(2-amino-2-phenylacetamido)-3-methyl-8-oxo-, D-
200. Ds-11971
201. B1692
202. Cefalexin, Vetranal(tm), Analytical Standard
203. C-2660
204. C06895
205. D00263
206. H10995
207. S-6437
208. Cephalexin, Antibiotic For Culture Media Use Only
209. Q411417
210. Q-200819
211. Brd-k90733503-002-03-6
212. Cefalexin, British Pharmacopoeia (bp) Reference Standard
213. Cephalexin Monohydrate, Antibiotic For Culture Media Use Only
214. Cephalexin, Pharmaceutical Secondary Standard; Certified Reference Material
215. 7-(d-.alpha.-amino-.alpha.-phenylacetamido)-3-methyl-3-cephem-4-carboxylic Acid
216. 7beta-[(2r)-2-amino-2-phenylacetamido]-3-methyl-3,4-didehydrocepham-4-carboxylic Acid
217. (6r,7r)-7-((r)-2-amino-2-phenyl-acetylamino)-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
218. (6r,7r)-7-((r)-2-amino-2-phenylacetamido)-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
219. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((aminophenylacetyl)amino)-3-methyl-8-oxo-,(6r-(6.alpha.,7.beta.(r*)))-
| Molecular Weight | 347.4 g/mol |
|---|---|
| Molecular Formula | C16H17N3O4S |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 347.09397721 g/mol |
| Monoisotopic Mass | 347.09397721 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Cephalexin |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cephalexin, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7- (D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4S H2O and the m... |
| Active Ingredient | Cephalexin |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 750mg base; eq 250mg base/5ml; eq 500mg base; eq 333mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Ranbaxy; Sun Pharm Inds (in); Belcher Pharms; Teva; Yung Shin Pharm; Alkem Labs; Aurobindo Pharma; Lupin; Hikma Pharms; Orchid Hlthcare; Hikma |
| 2 of 4 | |
|---|---|
| Drug Name | Keflex |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Keflex Capsules (Cephalexin, USP) is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4... |
| Active Ingredient | Cephalexin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Shionogi |
| 3 of 4 | |
|---|---|
| Drug Name | Cephalexin |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Cephalexin, USP is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7- (D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4S H2O and the m... |
| Active Ingredient | Cephalexin |
| Dosage Form | Tablet; Capsule; For suspension |
| Route | Oral |
| Strength | eq 750mg base; eq 250mg base/5ml; eq 500mg base; eq 333mg base; eq 250mg base; eq 125mg base/5ml |
| Market Status | Prescription |
| Company | Ranbaxy; Sun Pharm Inds (in); Belcher Pharms; Teva; Yung Shin Pharm; Alkem Labs; Aurobindo Pharma; Lupin; Hikma Pharms; Orchid Hlthcare; Hikma |
| 4 of 4 | |
|---|---|
| Drug Name | Keflex |
| PubMed Health | Cephalexin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | Keflex Capsules (Cephalexin, USP) is a semisynthetic cephalosporin antibiotic intended for oral administration. It is 7-(D--Amino--phenylacetamido)-3-methyl-3-cephem-4-carboxylic acid monohydrate. Cephalexin has the molecular formula C16H17N3O4... |
| Active Ingredient | Cephalexin |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 750mg base; eq 500mg base; eq 250mg base |
| Market Status | Prescription |
| Company | Shionogi |
Cephalosporins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/CEPHALEXIN/ HAS ANTIBACTERIAL SPECTRUM SIMILAR TO THAT OF PENICILLINS... AGAINST COCCI & GRAM-POSITIVE BACILLI, PENICILLIN G IS USUALLY MORE EFFECTIVE. ...MOST PENICILLINASES DO NOT AFFECT CEPHALEXIN...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1119
...CEPHALOSPORINS ARE HIGHLY EFFECTIVE IN THERAPY OF VARIETY OF MILD-TO-SEVERE INFECTIONS DUE TO BOTH GRAM-POSITIVE & GRAM-NEGATIVE MICROORGANISMS. /CEPHALOSPORINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1088
...CEPHALOSPORIN IS...DRUG OF 1ST CHOICE...FOR KLEBSIELLA INFECTIONS. ... THEY ARE...VALUABLE SECONDARY AGENTS, & THEY FREQUENTLY APPEAR AS ALTERNATIVE CHOICES TO PENICILLIN. /CEPHALOSPORINS/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1091
For more Therapeutic Uses (Complete) data for CEPHALEXIN (9 total), please visit the HSDB record page.
PHYSICIAN MUST ALTER EITHER DRUG DOSAGE OR INTERVAL BETWEEN DOSES WHEN RENAL FUNCTION IS IMPAIRED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALOSPORINS SHOULD NOT BE USED TO TREAT BACTERIAL MENINGITIS. THIS IS TRUE FOR ALL CAUSATIVE MICROORGANISMS. ...PENETRATION OF CEPHALOSPORINS INTO CSF IS POOR. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1164
INFECTIONS DUE TO ENTEROCOCCI ARE USUALLY UNAFFECTED BY THESE CMPD... ENTEROCOCCAL ENDOCARDITIS CANNOT BE CURED WITH CEPHALOSPORIN EVEN WHEN IT IS GIVEN CONCURRENTLY WITH GENTAMICIN OR STREPTOMYCIN. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
ENTEROBACTER (AEROBACTER) INFECTIONS ARE, AS A RULE, RESISTANT TO THESE CMPD. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
For more Drug Warnings (Complete) data for CEPHALEXIN (20 total), please visit the HSDB record page.
Cephalexin is indicated for the treatment of certain infections caused by susceptible bacteria. These infections include respiratory tract infections, otitis media, skin and skin structure infections, bone infections, and genitourinary tract infections.
FDA Label
Cephalexin (also called Cefalexin) is a first generation cephalosporin antibiotic. It is one of the most widely prescribed antibiotics, often used for the treatment of superficial infections that result as complications of minor wounds or lacerations. It is effective against most gram-positive bacteria through its inihibition of the cross linking reaction between N-acetyl muramicacid and N-acetylglucosamine in the cell wall, leading to cell lysis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB01 - Cefalexin
Absorption
Well absorbed from the upper gastrointestinal tract with nearly 100% oral bioavailability. Cephalexin is not absorbed in the stomach but is absorbed in the upper intestine. Patients taking 250mg of cephalexin reach a maximum plasma concentration of 7.7mcg/mL and patients taking 500mg reach 12.3mcg/mL.
Route of Elimination
Cephalexin is over 90% excreted in the urine after 6 hours by glomerular filtration and tubular secretion with a mean urinary recovery of 99.3%. Cephalexin is unchanged in the urine.
Volume of Distribution
5.2-5.8L.
Clearance
Clearance from one subject was 376mL/min.
LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY... MORE THAN 90%...IS EXCRETED UNALTERED IN URINE WITHIN 6 HR, PRIMARILY BY RENAL TUBULAR SECRETION. ...THERAPEUTICALLY EFFECTIVE CONCN ARE STILL ACHIEVED IN URINE OF PT WITH DECR RENAL FUNCTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALEXIN...IS WELL ABSORBED FROM GI TRACT. PEAK PLASMA CONCN, REACHED @ ABOUT 1 HR AFTER INGESTION OF DRUG, ARE APPROX 9 & 18 UG/ML AFTER ORAL DOSES OF 250 & 500 MG, RESPECTIVELY. INGESTION OF FOOD MAY DELAY ABSORPTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
CEPHALEXIN IS ALSO EXCRETED INTO BILE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
BOTH ABSORPTION & EXCRETION OF CEPHALEXIN ARE IMPAIRED IN NEW-BORN INFANTS, WHERE 24-HR URINARY RECOVERY OF ANTIBIOTIC ACCOUNTED FOR 5-66% OF DAILY ORAL DOSE.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 177
For more Absorption, Distribution and Excretion (Complete) data for CEPHALEXIN (14 total), please visit the HSDB record page.
Cephalexin is not metabolized in the body.
The half life of cephalexin is 49.5 minutes in a fasted state and 76.5 minutes with food though these times were not significantly different in the study.
LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY, T/2 OF CEPHALEXIN NORMALLY BEING ABOUT 40 MIN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
/IN RATS/ RATIOS OF BONE TO SERUM CONCN AVG...1:9 FOR CEPHALEXIN DURING 0.25-4 HR AFTER /ORAL/ DOSING. DESPITE DIFFERENCES IN CONCN; T/2 IN BONE & SERUM WERE SIMILAR.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 452
PEAK TIME, T/2 OF ELIMINATION, T/2 OF ABSORPTION, & VOL OF DISTRIBUTION WERE ALL SIMILAR FOLOWING ADMIN OF EITHER 1 OR 2 G OF CEPHALEXIN.
PMID:438352 CHOW M ET AL; J CLIN PHARMACOL 19 (4): 185-94 (1979)
The serum half-life of cephalexin is 0.5-1.2 hr in adults with normal renal function. The serum half-life of the drug is reported to be about 5 hr in neonates and 2.5 hr in children 3-12 mo of age. In one study, the serum half-life was 7.7 hr in adults with creatinine clearances of 9.2 ml/min and 13.9 hr in adults with creatinine clearances of 4 ml/min.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 166
Cephalexin is a first generation cephalosporin antibiotic. Cephalosporins contain a beta lactam and dihydrothiazide. Unlike penicillins, cephalosprins are more resistant to the action of beta lactamase. Cephalexin inhibits bacterial cell wall synthesis, leading breakdown and eventualy cell death.
CEPHALOTHIN & ITS CONGENERS INHIBIT BACTERIAL CELL-WALL SYNTHESIS IN MANNER SIMILAR TO THAT OF PENICILLIN. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
The penicillins and their metabolites are potent immunogens because of their ability to combine with proteins and act as haptens for acute antibody-mediated reactions. The most frequent (about 95 percent) or "major" determinant of penicillin allergy is the penicilloyl determinant produced by opening the beta-lactam ring of the penicillin. This allows linkage of the penicillin to protein at the amide group. "Minor" determinants (less frequent) are the other metabolites formed, including native penicillin and penicilloic acids. /Penicillins/
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 953
Bactericidal; action depends on ability to reach and bind penicillin-binding proteins located in bacterial cytoplasmic membranes; cephalosporins inhibit bacterial septum and cell wall synthesis, probably by acylation of membrane-bound transpeptidase enzymes. This prevents cross-linkage of peptidoglycan chains, which is necessary for bacterial cell wall strength and rigidity. Also, cell division and growth are inhibited, and lysis and elongation of susceptible bacteria frequently occur. Rapidly dividing bacteria are those most susceptible to the action of cephalosporins. /Cephalosporins/
USP Convention. USPDI - Drug Information for the Health Care Professional. 15 th ed. Volume 1. Rockville, MD: United States Pharmacopeial Convention, Inc., 1995. (Plus updates.), p. 679
CEPHALOSPORIN C IS VERY RESISTANT TO ACTION OF PENICILLINASE, FOR WHICH IT IS BOTH COMPETITIVE & NONCOMPETITIVE INHIBITOR, DEPENDING ON SUBSTRATE TESTED... /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13719
Submission : 1998-09-01
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13759
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18361
Submission : 2005-05-20
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13781
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13610
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3118
Submission : 1977-11-17
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23872
Submission : 2010-06-10
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13720
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9889
Submission : 1992-09-21
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13719
Submission : 1998-09-01
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13723
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13720
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9889
Submission : 1992-09-21
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13610
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1820
Submission : 1971-11-10
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13752
Submission : 1998-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3118
Submission : 1977-11-17
Status : Inactive
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13733
Submission : 1998-09-01
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 13715
Submission : 1998-09-01
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
About the Company : We can help your company solve the payment problems during the trading from chinese factories. We supply you with OEM from china.We are professsionals with the competitive price an...

About the Company : Located in Jinan, Qilu Pharmaceutical is one of the leading pharmaceutical companies in China. It focuses on developing, manufacturing and marketing of generic drugs and active pha...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 750MG BASE
Packaging :
Approval Date : 2023-12-06
Application Number : 65253
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 500MG BASE
Packaging :
Approval Date : 1987-04-22
Application Number : 62775
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Oral Suspension
Dosage Strength : 250mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Belex 500
Dosage Form : CAP
Dosage Strength : 500mg
Packaging : 100X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : DRY SYRUP
Dosage Strength : 125MG/5ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : PMS-CEPHALEXIN - TAB 500MG
Dosage Form : TABLET
Dosage Strength : 500MG
Packaging : 100 TABLETS
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : PMS-CEPHALEXIN 250 - SUS 250MG/5ML
Dosage Form : SUSPENSION
Dosage Strength : 2505MG/5ML
Packaging : 100/200ML
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : KEFLEX
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 125MG BASE/5ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 50406
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : CEPHALEXIN
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 125MG BASE/5ML
Packaging :
Approval Date : 2001-07-27
Application Number : 65081
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 250MG BASE
Packaging :
Approval Date : 1987-04-24
Application Number : 62760
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 125MG BASE/5ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons*
Approval Date : 1982-01-01
Application Number : 62117
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 250MG BASE
Approval Date : 2005-06-28
Application Number : 65248
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CEPHALEXIN
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 500MG BASE
Approval Date : 2024-09-27
Application Number : 218817
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 250MG BASE
Approval Date : 1988-07-15
Application Number : 62713
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 250MG BASE/5ML
Approval Date : 1989-07-25
Application Number : 62987
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 500MG BASE
Approval Date : 1988-03-17
Application Number : 62869
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : KEFLEX
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 250MG BASE/5ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons*
Approval Date : 1982-01-01
Application Number : 50406
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 250MG BASE
Approval Date : 1987-06-11
Application Number : 62791
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : CAPSULE;ORAL
Dosage Strength : EQ 500MG BASE
Approval Date : 1999-09-16
Application Number : 65007
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : CEPHALEXIN
Dosage Form : FOR SUSPENSION;ORAL
Dosage Strength : EQ 125MG BASE/5ML
Approval Date : 1987-06-16
Application Number : 62767
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : Italy
Brand Name : Keforal
Dosage Form : Cephalexin 5% 100Ml Oral Use
Dosage Strength : grat os suspe 100 ml 5%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Keforal
Dosage Form : Cephalexin 500Mg 8 Joined' Oral Use
Dosage Strength : 8 cpr riv 500 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Keforal
Dosage Form : Cephalexin 1.000Mg 8 Joined' Oral Use
Dosage Strength : 8 cpr riv 1 g
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Italy
Brand Name : Ceporex
Dosage Form : Cephalexin 1.000Mg 8 Joined' Oral Use
Dosage Strength : 8 cpr riv 1 g
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Normon Cephalexin 500Mg 28 Capsules
Dosage Form : Capsule
Dosage Strength : 500 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info :
Registration Country : Spain
Brand Name : Kefloridina Forte 500Mg 28 Capsules
Dosage Form : Capsule
Dosage Strength : 500 Mg/Capsule
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Spain

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
55
PharmaCompass offers a list of Cephalexin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Cephalexin manufacturer or Cephalexin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Cephalexin manufacturer or Cephalexin supplier.
PharmaCompass also assists you with knowing the Cephalexin API Price utilized in the formulation of products. Cephalexin API Price is not always fixed or binding as the Cephalexin Price is obtained through a variety of data sources. The Cephalexin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Cephalexin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Cephalexin, including repackagers and relabelers. The FDA regulates Cephalexin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Cephalexin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Cephalexin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Cephalexin supplier is an individual or a company that provides Cephalexin active pharmaceutical ingredient (API) or Cephalexin finished formulations upon request. The Cephalexin suppliers may include Cephalexin API manufacturers, exporters, distributors and traders.
click here to find a list of Cephalexin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Cephalexin DMF (Drug Master File) is a document detailing the whole manufacturing process of Cephalexin active pharmaceutical ingredient (API) in detail. Different forms of Cephalexin DMFs exist exist since differing nations have different regulations, such as Cephalexin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Cephalexin DMF submitted to regulatory agencies in the US is known as a USDMF. Cephalexin USDMF includes data on Cephalexin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Cephalexin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Cephalexin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Cephalexin Drug Master File in Japan (Cephalexin JDMF) empowers Cephalexin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Cephalexin JDMF during the approval evaluation for pharmaceutical products. At the time of Cephalexin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Cephalexin suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Cephalexin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Cephalexin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Cephalexin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Cephalexin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Cephalexin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Cephalexin suppliers with NDC on PharmaCompass.
Cephalexin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Cephalexin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Cephalexin GMP manufacturer or Cephalexin GMP API supplier for your needs.
A Cephalexin CoA (Certificate of Analysis) is a formal document that attests to Cephalexin's compliance with Cephalexin specifications and serves as a tool for batch-level quality control.
Cephalexin CoA mostly includes findings from lab analyses of a specific batch. For each Cephalexin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Cephalexin may be tested according to a variety of international standards, such as European Pharmacopoeia (Cephalexin EP), Cephalexin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Cephalexin USP).
