
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
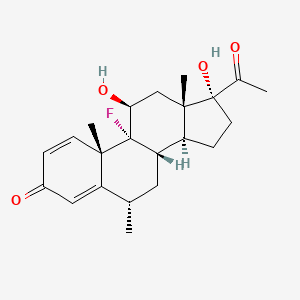

1. Cortisdin
2. Efflumidex
3. Flucon
4. Flucon, Isopto
5. Fluor Op
6. Fluor-op
7. Fluoro Ophtal
8. Fluoro-ophtal
9. Fluoropos
10. Fml
11. Fml Forte
12. Fml Liquifilm
13. Isopto Flucon
14. Pms Fluorometholone
15. Pms-fluorometholone
1. 426-13-1
2. Fluoromethalone
3. Oxylone
4. Flumetholon
5. Fluor-op
6. Fluormetholone
7. Cortilet
8. Delmeson
9. Fml Liquifilm
10. Trilcin
11. Fml Forte
12. Fluorometolona
13. Fluorometholonum
14. Fluormetholon
15. Nsc 33001
16. Fml
17. Component Of Neo-oxylone
18. U 8614
19. Chebi:31625
20. 9-fluoro-11beta,17-dihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
21. Nsc-33001
22. (6s,8s,9r,10s,11s,13s,14s,17r)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
23. Sv0csg527l
24. Mls000069537
25. Mls001076157
26. Fluormetholonum
27. 9-fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione
28. Nsc33001
29. Fluorometolone
30. Smr000058598
31. Fluorometolone [dcit]
32. Neo-oxylone
33. Fml-s Liquifilm
34. Dsstox_cid_27435
35. Dsstox_rid_82345
36. Fml S.o.p.
37. Dsstox_gsid_47435
38. Fluorometholonum [inn-latin]
39. Fluorometolona [inn-spanish]
40. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha.,11.beta.)-
41. Oxylone (tn)
42. Fluor-op (tn)
43. Fml (tn)
44. Einecs 207-041-5
45. Unii-sv0csg527l
46. Fluorometholon
47. Ai3-52813
48. Ncgc00016442-01
49. Cas-426-13-1
50. Prestwick_227
51. 9-fluoro-11-beta,17-dihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione
52. Fluorometholone [usp:inn:ban:jan]
53. Mfcd00056461
54. Opera_id_341
55. Prestwick0_000718
56. Prestwick1_000718
57. Prestwick2_000718
58. Prestwick3_000718
59. F0414
60. 11beta,17alpha-dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione
61. Fluorometholone, >=98%
62. Pregna-1,4-diene-3,20-dione, 9-fluoro-11-beta,17-dihydroxy-6-alpha-methyl-
63. Schembl5051
64. Fluorometholone [mi]
65. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6alpha,11beta)-
66. Bspbio_000935
67. Fluorometholone [inn]
68. Fluorometholone [jan]
69. Spbio_002856
70. Fluorometholone [vandf]
71. Bpbio1_001029
72. Gtpl7079
73. Fluorometholone [mart.]
74. Chembl1200600
75. Dtxsid7047435
76. Fluorometholone [usp-rs]
77. Fluorometholone [who-dd]
78. 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methylpregna-1,4-diene-3,20-dione
79. Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methyl-
80. Fluorometholone (jp17/usp/inn)
81. Hms1570o17
82. Hms2097o17
83. Hms2234f16
84. Hms3714o17
85. Hy-b1893
86. Tox21_110440
87. Tox21_302593
88. Bdbm50103631
89. Fluorometholone [orange Book]
90. S5486
91. Akos015895108
92. Fml-s Component Fluorometholone
93. Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17-dihydroxy-6alpha-methyl-
94. Tox21_110440_1
95. Zinc118912517
96. Ac-3520
97. Ccg-220718
98. Db00324
99. Fluorometholone [usp Monograph]
100. (6alpha,11beta)-9-fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione
101. Ncgc00021575-03
102. Ncgc00021575-05
103. Ncgc00256631-01
104. As-12363
105. Fluorometholone Component Of Fml-s
106. Nci60_002886
107. Cs-0013955
108. Progesterone, 17-dihydroxy-6.alpha.-methyl-
109. D01367
110. 426f131
111. Q607349
112. Sr-01000003019
113. Sr-01000003019-2
114. Brd-k64862097-001-03-9
115. Brd-k64862097-001-12-0
116. Fluorometholone, British Pharmacopoeia (bp) Reference Standard
117. Fluorometholone, United States Pharmacopeia (usp) Reference Standard
118. Pregna-1,20-dione, 9-fluoro-11.beta.,17-dihydroxy-6.alpha.-methyl-
119. Progesterone, 1-dehydro-9-fluoro-11.beta., 17-dihydroxy-6.alpha.-methyl-
120. Pregna-1,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha.,11.beta.)-
121. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6i+/-,11i(2))-
122. (1r,2s,8s,10s,11s,14r,15s,17s)-14-acetyl-1-fluoro-14,17-dihydroxy-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one
123. (1r,2s,8s,10s,11s,14r,15s,17s)-14-acetyl-1-fluoro-14,17-dihydroxy-2,8,15-trimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one
124. Pregna-1, 4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-6-methyl-, (6.alpha., 11.beta.)-
| Molecular Weight | 376.5 g/mol |
|---|---|
| Molecular Formula | C22H29FO4 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 376.20498756 g/mol |
| Monoisotopic Mass | 376.20498756 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 787 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Fml |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML (fluorometholone ophthalmic ointment) 0.1% is a sterile, topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 4 | |
|---|---|
| Drug Name | Fml forte |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML FORTE (fluorometholone ophthalmic suspension, USP) 0.25% is a sterile, topical anti-inflammatory agent for ophthalmic use. Chemical NameFluorometholone: 9-Fluoro-11, 17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione. Structural FormulaContai... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 4 | |
|---|---|
| Drug Name | Fml |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML (fluorometholone ophthalmic ointment) 0.1% is a sterile, topical anti-inflammatory agent for ophthalmic use.... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Ointment; Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.1% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 4 | |
|---|---|
| Drug Name | Fml forte |
| PubMed Health | Fluorometholone (Into the eye) |
| Drug Classes | Ophthalmologic Agent |
| Drug Label | FML FORTE (fluorometholone ophthalmic suspension, USP) 0.25% is a sterile, topical anti-inflammatory agent for ophthalmic use. Chemical NameFluorometholone: 9-Fluoro-11, 17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione. Structural FormulaContai... |
| Active Ingredient | Fluorometholone |
| Dosage Form | Suspension/drops |
| Route | Ophthalmic |
| Strength | 0.25% |
| Market Status | Prescription |
| Company | Allergan |
For the ophthalmic treatment of corticosteroid-responsive inflammation of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe.
FDA Label
Corticosteroids such as fluorometholone inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
S01BA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C05 - Vasoprotectives
C05A - Agents for treatment of hemorrhoids and anal fissures for topical use
C05AA - Corticosteroids
C05AA06 - Fluorometholone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AB - Corticosteroids, moderately potent (group ii)
D07AB06 - Fluorometholone
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07X - Corticosteroids, other combinations
D07XB - Corticosteroids, moderately potent, other combinations
D07XB04 - Fluorometholone
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AA - Corticosteroids, combinations for treatment of acne
D10AA01 - Fluorometholone
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA07 - Fluorometholone
S - Sensory organs
S01 - Ophthalmologicals
S01C - Antiinflammatory agents and antiinfectives in combination
S01CB - Corticosteroids/antiinfectives/mydriatics in combination
S01CB05 - Fluorometholone
There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. Their primary target is the cytosolic glucocorticoid receptor. After binding the receptor the newly formed receptor-ligand complex translocates itself into the cell nucleus, where it binds to many glucocorticoid response elements (GRE) in the promoter region of the target genes. The DNA bound receptor then interacts with basic transcription factors, causing the increase in expression of specific target genes.
GDUFA
DMF Review : Complete
Rev. Date : 2024-07-18
Pay. Date : 2024-06-12
DMF Number : 35780
Submission : 2021-04-13
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2014-04-18
Pay. Date : 2014-04-11
DMF Number : 4423
Submission : 1982-02-03
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3108
Submission : 1978-01-16
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-03-03
Pay. Date : 2022-02-16
DMF Number : 36772
Submission : 2022-02-08
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37674
Submission : 2023-01-03
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 1541
Submission : 1970-06-25
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4761
Submission : 1982-12-17
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 218MF10387
Registrant's Address : 15 rue Traversie(')re 75012 Paris France
Initial Date of Registration : 2006-03-20
Latest Date of Registration : 2016-12-20
Registration Number : 306MF10063
Registrant's Address : Parque Tecnologico-Parcela 105 Boecillo (Valladolid) Spain
Initial Date of Registration : 2024-05-08
Latest Date of Registration : 2024-05-08

Registration Number : 303MF10110
Registrant's Address : Via de Amicis 47, 20123 Milano, Italy
Initial Date of Registration : 2021-07-16
Latest Date of Registration : 2021-07-16

Registration Number : 218MF10820
Registrant's Address : Piazzale Luigi Cadorna, 4 - 20123 MILANO, Italy
Initial Date of Registration : 2006-10-06
Latest Date of Registration : 2006-10-06

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Symbiotec, a leading API manufacturing company based in Indore, Central India, specializes in Cortico-Steroids and Steroid-Hormone APIs. Since 1995, their focus on R&D, sustainable...
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
About the Company : Gonane Pharma, is a contract pharmaceutical company located in Gujarat, India, specializing in the manufacturing and marketing of Corticosteroids, Hormones, Antivirals, and Oncolog...
About the Company : Axplora, created from the merger of Farmabios, Novasep & PharmaZell, is a leading API manufacturing partner to the world’s leading pharma & biotech companies, delivering top-qual...
About the Company : EUROAPI is the market leader in small molecule APIs with projected sales of about €1 billion in 2022. With around 200 APIs, it has one of the largest portfolios in the market. Th...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long-standing tradition of advancing health through innovation and dedication. Today, we proudly build upon this legacy, drivi...
About the Company : Curia is a global contract research, development and manufacturing organization (CDMO), offering products and services across the drug development spectrum to help our partners tur...

About the Company : Guangzhou Tosun Pharmaceutical was founded in 1999, which mainly focuses on importation & exportation of Active Pharmaceutical Ingrediants, Chemical Raw Materials, Intermediate, Ex...

About the Company : Founded in 2005, Hangzhou Deli Chemical Co., Ltd. is a specialized manufacturing factory of APIs, pharmaceutical intermediates and other chemicals, with production bases in Huzhou,...

About the Company : Human Pharmalabs Pvt. Ltd. was founded to manufacture lifesaving critical APIs in India, pioneering the introduction of Corticosteroid APIs. The company adheres to international st...

About the Company : Maharshi Pharmachem is the fastest growing company in India, having plans to expand our market reach further. We have the conviction that all markets should be provided with qualit...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
FML suspension (fluorometholone) is a glucocorticoid receptor agonist, with its generic approved by USFDA for corticosteroid-responsive inflammation of the conjunctiva, cornea, and anterior segment.
Lead Product(s): Fluorometholone
Therapeutic Area: Ophthalmology Brand Name: Fluorometholone-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fluorometholone
Therapeutic Area : Ophthalmology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Amneal Launches Complex Generic Fluorometholone Ophthalmic Suspension
Details : FML suspension (fluorometholone) is a glucocorticoid receptor agonist, with its generic approved by USFDA for corticosteroid-responsive inflammation of the conjunctiva, cornea, and anterior segment.
Product Name : Fluorometholone-Generic
Product Type : Small molecule
Upfront Cash : Not Applicable
January 10, 2024

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]21-Desacetoxy Anecortave Oxediene
CAS Number : 34184-82-2
End Use API : Fluorometholone
About The Company : EUROAPI is the market leader in small molecule APIs with projected sales of about €1 billion in 2022. With around 200 APIs, it has one of the largest portfoli...
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Novomix is used as a ready mix film coating system in the production of pharmaceutical tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
38
PharmaCompass offers a list of Fluorometholone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Fluorometholone manufacturer or Fluorometholone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Fluorometholone manufacturer or Fluorometholone supplier.
PharmaCompass also assists you with knowing the Fluorometholone API Price utilized in the formulation of products. Fluorometholone API Price is not always fixed or binding as the Fluorometholone Price is obtained through a variety of data sources. The Fluorometholone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluorometholon manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluorometholon, including repackagers and relabelers. The FDA regulates Fluorometholon manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluorometholon API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Fluorometholon manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Fluorometholon supplier is an individual or a company that provides Fluorometholon active pharmaceutical ingredient (API) or Fluorometholon finished formulations upon request. The Fluorometholon suppliers may include Fluorometholon API manufacturers, exporters, distributors and traders.
click here to find a list of Fluorometholon suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Fluorometholon DMF (Drug Master File) is a document detailing the whole manufacturing process of Fluorometholon active pharmaceutical ingredient (API) in detail. Different forms of Fluorometholon DMFs exist exist since differing nations have different regulations, such as Fluorometholon USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Fluorometholon DMF submitted to regulatory agencies in the US is known as a USDMF. Fluorometholon USDMF includes data on Fluorometholon's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Fluorometholon USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Fluorometholon suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Fluorometholon Drug Master File in Japan (Fluorometholon JDMF) empowers Fluorometholon API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Fluorometholon JDMF during the approval evaluation for pharmaceutical products. At the time of Fluorometholon JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Fluorometholon suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Fluorometholon Drug Master File in Korea (Fluorometholon KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Fluorometholon. The MFDS reviews the Fluorometholon KDMF as part of the drug registration process and uses the information provided in the Fluorometholon KDMF to evaluate the safety and efficacy of the drug.
After submitting a Fluorometholon KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Fluorometholon API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Fluorometholon suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Fluorometholon as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Fluorometholon API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Fluorometholon as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Fluorometholon and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Fluorometholon NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Fluorometholon suppliers with NDC on PharmaCompass.
Fluorometholon Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluorometholon GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluorometholon GMP manufacturer or Fluorometholon GMP API supplier for your needs.
A Fluorometholon CoA (Certificate of Analysis) is a formal document that attests to Fluorometholon's compliance with Fluorometholon specifications and serves as a tool for batch-level quality control.
Fluorometholon CoA mostly includes findings from lab analyses of a specific batch. For each Fluorometholon CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluorometholon may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluorometholon EP), Fluorometholon JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluorometholon USP).
