
Synopsis
Synopsis
0
EU WC
0
NDC API
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
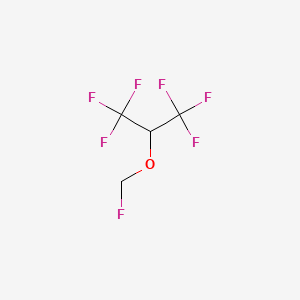

1. Bax 3084
2. Fluoromethyl Hexafluoroisopropyl Ether
3. Fluoromethyl-2,2,2-trifluoro-1-(trifluoromethyl)ethyl Ether
4. Sevorane
5. Ultane
1. 28523-86-6
2. Ultane
3. Sevofluran
4. 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane
5. Sevorane
6. Sojourn
7. Mr6s4
8. Sevoflo
9. Sevofluranum
10. Sevoflurano
11. Sevofluranum [inn-latin]
12. Sevoflurano [inn-spanish]
13. Fluoromethyl 1,1,1,3,3,3-hexafluoroisopropyl Ether
14. Sevocalm
15. Propane, 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)-
16. Mr-6s4
17. Fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl Ether
18. Nsc-760367
19. 38lvp0k73a
20. Chebi:9130
21. Propane,1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)-
22. Ncgc00167421-01
23. Sevofrane
24. Bax 3084
25. Petrem
26. Fluoromethyl 1,1,1,3,3,3-hexafluoroisopropyl Ether (sevoflurane)
27. Ultane (tn)
28. Brn 2041023
29. Unii-38lvp0k73a
30. Hsdb 8059
31. Mfcd00153189
32. Sevoflurane [usan:usp:inn:ban:jan]
33. Sevoflurane [mi]
34. Sevoflurane [inn]
35. Sevoflurane [jan]
36. F0691
37. Sevoflurane [usan]
38. Sevoflurane [vandf]
39. (cf3)2choch2f
40. Dsstox_cid_26614
41. Dsstox_rid_81767
42. Sevoflurane [mart.]
43. Dsstox_gsid_46614
44. Schembl61918
45. Sevoflurane [usp-rs]
46. Sevoflurane [who-dd]
47. Gtpl7296
48. Chembl1200694
49. Dtxsid8046614
50. Sevoflurane [green Book]
51. Sevoflurane (jp17/usan/inn)
52. Sevoflurane [ep Impurity]
53. Sevoflurane [orange Book]
54. Hms3264n21
55. Pharmakon1600-01503680
56. Sevoflurane [ep Monograph]
57. Sevoflurane [usp Impurity]
58. Sevoflurane [usp Monograph]
59. Zinc1530810
60. Tox21_112425
61. Ether, Fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl-
62. Nsc760367
63. Akos007930500
64. Ccg-213707
65. Db01236
66. Nsc 760367
67. Ncgc00167421-02
68. Sevoflurane [ema Epar Veterinary]
69. Ac-15484
70. As-13261
71. Fluoromethyl 2h-hexafluoroprop-2-yl Ether
72. Cas-28523-86-6
73. Db-047409
74. Ft-0605909
75. S2464
76. 6-chlorobenzimidazole-4-carboxylicacid
77. C07520
78. D00547
79. D78401
80. Ab01563174_01
81. 523f866
82. A819479
83. Q419394
84. Sr-01000944968
85. J-524240
86. Sr-01000944968-1
87. 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane;sevoflurane
| Molecular Weight | 200.05 g/mol |
|---|---|
| Molecular Formula | C4H3F7O |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 200.00721185 g/mol |
| Monoisotopic Mass | 200.00721185 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Sevoflurane |
| PubMed Health | Sevoflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | Sevoflurane, USP, volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane, USP is fluoromethyl 2,2,2,-trifluoro-1-(trifluoromethyl) ethyl et... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Halocarbon Prods |
| 2 of 6 | |
|---|---|
| Drug Name | Sojourn |
| PubMed Health | Sevoflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | Sojourn (sevoflurane, USP), a volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane is fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Piramal Critical |
| 3 of 6 | |
|---|---|
| Drug Name | Ultane |
| Drug Label | ULTANE (sevoflurane), volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane is fluoromethyl 2,2,2,-trifluoro-1-(trifluoromethyl) ethyl eth... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Abbvie |
| 4 of 6 | |
|---|---|
| Drug Name | Sevoflurane |
| PubMed Health | Sevoflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | Sevoflurane, USP, volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane, USP is fluoromethyl 2,2,2,-trifluoro-1-(trifluoromethyl) ethyl et... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Baxter Hlthcare; Halocarbon Prods |
| 5 of 6 | |
|---|---|
| Drug Name | Sojourn |
| PubMed Health | Sevoflurane (By breathing) |
| Drug Classes | Volatile Liquid |
| Drug Label | Sojourn (sevoflurane, USP), a volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane is fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Piramal Critical |
| 6 of 6 | |
|---|---|
| Drug Name | Ultane |
| Drug Label | ULTANE (sevoflurane), volatile liquid for inhalation, a nonflammable and nonexplosive liquid administered by vaporization, is a halogenated general inhalation anesthetic drug. Sevoflurane is fluoromethyl 2,2,2,-trifluoro-1-(trifluoromethyl) ethyl eth... |
| Active Ingredient | Sevoflurane |
| Dosage Form | Liquid |
| Route | Inhalation |
| Strength | 100% |
| Market Status | Prescription |
| Company | Abbvie |
Sevoflurane is an inhalational anesthetic agent for use in induction and maintenance of general anesthesia. Minimum alveolar concentration (MAC) of sevoflurane in oxygen for a 40-year-old adult is 2.1%. The MAC of sevoflurane decreases with age. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Sevoflurane (n = 29) was compared to isoflurane (n = 27) in ASA Class I or II patients for the maintenance of anesthesia during cesarean section. Newborn evaluations and recovery events were recorded. With both anesthetics, Apgar scores averaged 8 and 9 at 1 and 5 minutes, respectively. Use of sevoflurane as part of general anesthesia for elective cesarean section produced no untoward effects in mother or neonate. ... /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Sevoflurane is indicated for induction and maintenance of general anesthesia in adult and pediatric patients for inpatient and outpatient surgery. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
/EXPERIMENTAL THER:/ Volatile anesthetic agents have been recognized for their neuroprotective properties since the 1960s. However, little is known regarding the potential retinoprotective effects of preconditioning by anesthetic drugs. Retinal ischemia can be modeled by permanent bilateral common carotid artery occlusion (BCCAO). /Investigators/ studied the degree of ischemic injury with preconditioning by sevoflurane in the rat retina. During the BCCAO operation and preconditioning Wistar rats were anesthetized with 1 MAC of sevoflurane. ... /Four groups were examined/: non- and preconditioning groups in control and BCCAO animals. The duration of preconditioning period was 1 hr and it was performed 1 day before BCCAO. The retinas were processed for histological evaluation after 2 weeks survival to determine the cell number in the ganglion cell layer and the thickness of the whole retina and that of all retinal layers. BCCAO-induced retinal ischemic injury was ameliorated by sevoflurane preconditioning. Retinal thickness and the cell number in the ganglion cell layer were more retained in preconditioned animals after BCCAO compared to non-preconditioned group. These results suggest that preconditioning using sevoflurane could provide a new perspective in retinoprotective strategies.
PMID:22684245 Szabadfi K et al; J Mol Histol 43 (5): 565-9 (2012)
For more Therapeutic Uses (Complete) data for Sevoflurane (7 total), please visit the HSDB record page.
Potassium hydroxide containing CO2 absorbents (e.g. BARALYME) are not recommended for use with sevoflurane, USP.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
VET: Infrequent adverse reactions include paddling, retching, salivation, cyanosis, premature ventricular contractions and excessive cardiopulmonary depression. Transient elevations in liver function tests and white blood cell count may occur with sevoflurane, USP, as with the use of other halogenated anesthetic agents.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
VET: The most frequently reported adverse reactions during maintenance anesthesia were hypotension, followed by tachypnea, muscle tenseness, excitation, apnea, muscle fasciculations and emesis.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
Although data from controlled clinical studies at low flow rates are limited, findings taken from patient and animal studies suggest that there is a potential for renal injury which is presumed due to Compound A. Animal and human studies demonstrate that sevoflurane administered for more than 2 MAC hours and at fresh gas flow rates of < 2 L/min may be associated with proteinuria and glycosuria.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
For more Drug Warnings (Complete) data for Sevoflurane (13 total), please visit the HSDB record page.
Used for induction and maintenance of general anesthesia in adult and pediatric patients for inpatient and outpatient surgery.
FDA Label
For the induction and maintenance of anaesthesia in dogs and cats.
Sevoflurane induces muscle relaxation and reduces pains sensitivity by altering tissue excitability with a fast onset of action. It does so by decreasing the extent of gap junction mediated cell-cell coupling and altering the activity of the channels that underlie the action potential.
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Anesthetics, Inhalation
Gases or volatile liquids that vary in the rate at which they induce anesthesia; potency; the degree of circulation, respiratory, or neuromuscular depression they produce; and analgesic effects. Inhalation anesthetics have advantages over intravenous agents in that the depth of anesthesia can be changed rapidly by altering the inhaled concentration. Because of their rapid elimination, any postoperative respiratory depression is of relatively short duration. (From AMA Drug Evaluations Annual, 1994, p173) (See all compounds classified as Anesthetics, Inhalation.)
QN01AB08
N01AB08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N01 - Anesthetics
N01A - Anesthetics, general
N01AB - Halogenated hydrocarbons
N01AB08 - Sevoflurane
Absorption
Rapidly absorbed into circulation via the lungs, however solubility in the blood is low.
Route of Elimination
The low solubility of sevoflurane facilitates rapid elimination via the lungs. In vivo metabolism studies suggest that approximately 5% of the sevoflurane dose may be metabolized. Up to 3.5% of the sevoflurane dose appears in the urine as inorganic fluoride.
Up to 3.5% of the sevoflurane dose appears in the urine as inorganic fluoride. Studies on fluoride indicate that up to 50% of fluoride clearance is nonrenal (via fluoride being taken up into bone).
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
The low solubility of sevoflurane facilitates rapid elimination via the lungs. The rate of elimination is quantified as the rate of change of the alveolar (end-tidal) concentration following termination of anesthesia (FA), relative to the last alveolar concentration (FaO) measured immediately before discontinuance of the anesthetic.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Fluoride ion concentrations are influenced by the duration of anesthesia, the concentration of sevoflurane administered, and the composition of the anesthetic gas mixture. In studies where anesthesia was maintained purely with sevoflurane for periods ranging from 1 to 6 hours, peak fluoride concentrations ranged between 12 uM and 90 uM. Peak concentrations occur within 2 hours of the end of anesthesia and are less than 25 uM (475 ng/mL) for the majority of the population after 10 hours.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
The concentrations of sevoflurane in milk are probably of no clinical importance 24 hours after anesthesia. Because of rapid washout, sevoflurane concentrations in milk are predicted to be below those found with many other volatile anesthetics.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Compared with healthy individuals, the fluoride ion half-life was prolonged in patients with renal impairment, but not in the elderly. A study in 8 patients with hepatic impairment suggests a slight prolongation of the half-life. The mean half-life in patients with renal impairment averaged approximately 33 hours (range 21-61 hours) as compared to a mean of approximately 21 hours (range 10-48 hours) in normal healthy individuals. The mean half-life in the elderly (greater than 65 years) approximated 24 hours (range 18-72 hours). The mean half-life in individuals with hepatic impairment was 23 hours (range 16-47 hours).
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Relatively little biotransformation, only 5% is metabolized by cytochrome P450 CYP2E1 to hexafluoroisopropanol (HFIP) with release of inorganic fluoride and CO2. No other metabolic pathways have been identified for sevoflurane.
Renal and hepatic toxicity of the fluorinated ether volatile anesthetics is caused by biotransformation to toxic metabolites. Metabolism also contributes significantly to the elimination pharmacokinetics of some volatile agents. Although innumerable studies have explored anesthetic metabolism in animals, there is little information on human volatile anesthetic metabolism with respect to comparative rates or the identity of the enzymes responsible for defluorination. The first purpose of this investigation was to compare the metabolism of the fluorinated ether anesthetics by human liver microsomes. The second purpose was to test the hypothesis that cytochrome P450 2E1 is the specific P450 isoform responsible for volatile anesthetic defluorination in humans. Microsomes were prepared from human livers. Anesthetic metabolism in microsomal incubations was measured by fluoride production. The strategy for evaluating the role of P450 2E1 in anesthetic defluorination involved three approaches: for a series of 12 human livers, correlation of microsomal defluorination rate with microsomal P450 2E1 content (measured by Western blot analysis), correlation of defluorination rate with microsomal P450 2E1 catalytic activity using marker substrates (para-nitrophenol hydroxylation and chlorzoxazone 6-hydroxylation), and chemical inhibition by P450 isoform-selective inhibitors. The rank order of anesthetic metabolism, assessed by fluoride production at saturating substrate concentrations, was methoxyflurane > sevoflurane > enflurane > isoflurane > desflurane > 0. There was a significant linear correlation of sevoflurane and methoxyflurane defluorination with antigenic P450 2E1 content (r = 0.98 and r = 0.72, respectively), but not with either P450 1A2 or P450 3A3/4. Comparison of anesthetic defluorination with either para-nitrophenol or chlorzoxazone hydroxylation showed a significant correlation for sevoflurane (r = 0.93, r = 0.95) and methoxyflurane (r = 0.78, r = 0.66). Sevoflurane defluorination was also highly correlated with that of enflurane (r = 0.93), which is known to be metabolized by human P450 2E1. Diethyldithiocarbamate, a selective inhibitor of P450 2E1, produced a concentration-dependent inhibition of sevoflurane, methoxyflurane, and isoflurane defluorination. No other isoform-selective inhibitor diminished the defluorination of sevoflurane, whereas methoxyflurane defluorination was inhibited by the selective P450 inhibitors furafylline (P450 1A2), sulfaphenazole (P450 2C9/10), and quinidine (P450 2D6) but to a much lesser extent than by diethyldithiocarbamate. These results demonstrate that cytochrome P450 2E1 is the principal, if not sole human liver microsomal enzyme catalyzing the defluorination of sevoflurane. P450 2E1 is the principal, but not exclusive enzyme responsible for the metabolism of methoxyflurane, which also appears to be catalyzed by P450s 1A2, 2C9/10, and 2D6. The data also suggest that P450 2E1 is responsible for a significant fraction of isoflurane metabolism. Identification of P450 2E1 as the major anesthetic metabolizing enzyme in humans provides a mechanistic understanding of clinical fluorinated ether anesthetic metabolism and toxicity.
PMID:8214760 Kharasch ED, Thummel KE; Anesthesiology 79 (4): 795-807 (1993)
Sevoflurane, USP is metabolized to hexafluoroisopropanol (HFIP) with release of inorganic fluoride and CO2. Fluoride ion concentrations are influenced by the duration of anesthesia and the concentration of sevoflurane, USP. Once formed, HFIP is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite. No other metabolic pathways for sevoflurane, USP have been identified. In humans, the fluoride ion half-life was prolonged in patients with renal impairment, but human clinical trials contained no reports of toxicity associated with elevated fluoride ion levels.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
Cytochrome P450 2E1 is the principal isoform identified for sevoflurane metabolism and this may be induced by chronic exposure to isoniazid and ethanol. This is similar to the metabolism of isoflurane and enflurane and is distinct from that of methoxyflurane which is metabolized via a variety of cytochrome P450 isoforms. The metabolism of sevoflurane is not inducible by barbiturates. As shown in Figure 5, inorganic fluoride concentrations peak within 2 hours of the end of sevoflurane anesthesia and return to baseline concentrations within 48 hours post-anesthesia in the majority of cases (67%). The rapid and extensive pulmonary elimination of sevoflurane minimizes the amount of anesthetic available for metabolism.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Sevoflurane is metabolized by cytochrome P450 2E1, to hexafluoroisopropanol (HFIP) with release of inorganic fluoride and CO2. Once formed HFIP is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite. No other metabolic pathways for sevoflurane have been identified. In vivo metabolism studies suggest that approximately 5% of the sevoflurane dose may be metabolized.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
In a study in which 4 dogs were exposed to 4% sevoflurane, USP for 3 hours, maximum serum fluoride concentrations of 17.0-27.0 umole/L were observed after 3 hours of anesthesia. Serum fluoride fell quickly after anesthesia ended, and had returned to baseline by 24 hours post-anesthesia.
US Natl Inst Health; DailyMed. Current Medication Information for PETREM (sevoflurane) injection, solution (June 2010). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ad274882-3c54-489f-bf78-c200b8ab348d
15-23 hours
Compared with healthy individuals, the fluoride ion half-life was prolonged in patients with renal impairment, but not in the elderly. A study in 8 patients with hepatic impairment suggests a slight prolongation of the half-life. The mean half-life in patients with renal impairment averaged approximately 33 hours (range 21-61 hours) as compared to a mean of approximately 21 hours (range 10-48 hours) in normal healthy individuals. The mean half-life in the elderly (greater than 65 years) approximated 24 hours (range 18-72 hours). The mean half-life in individuals with hepatic impairment was 23 hours (range 16-47 hours).
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
The half-life is in the range of 15-23 hours.
US Natl Inst Health; DailyMed. Current Medication Information for ULTANE (sevoflurane) liquid (May 2011). Available from, as of June 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c6e76bc-c964-4955-e0a3-511d3386a9cc
Sevoflurane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times. Sevoflurane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane. It also appears to bind the D subunit of ATP synthase and NADH dehydogenase and also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor, and the glycine receptor.
Sevoflurane is widely used as a volatile anesthetic in clinical practice. However, its mechanism is still unclear. ...It has been reported that voltage-gated sodium channels have important roles in anesthetic mechanisms. Much attention has been paid to the effects of sevoflurane on voltage-dependent sodium channels. To elucidate this, /investigators/ examined the effects of sevoflurane on Na(v) 1.8, Na(v) 1.4, and Na(v) 1.7 expressed in Xenopus oocytes. The effects of sevoflurane on Na(v) 1.8, Na(v) 1.4, and Na(v) 1.7 sodium channels were studied by an electrophysiology method using whole-cell, two-electrode voltage-clamp techniques in Xenopus oocytes. Sevoflurane at 1.0 mM inhibited the voltage-gated sodium channels Na(v)1.8, Na(v)1.4, and Na(v)1.7, but sevoflurane (0.5 mM) had little effect. This inhibitory effect of 1 mM sevoflurane was completely abolished by pretreatment with protein kinase C (PKC) inhibitor, bisindolylmaleimide I. Sevoflurane appears to have inhibitory effects on Na(v)1.8, Na(v)1.4, and Na(v) 1.7 by PKC pathways. However, these sodium channels might not be related to the clinical anesthetic effects of sevoflurane.
PMID:21656091 Yokoyama T et al; J Anesth 25 (4): 609-13 (2011)
Sevoflurane has been demonstrated to vasodilate the feto-placental vasculature. /Investigators/ aimed to determine the contribution of modulation of potassium and calcium channel function to the vasodilatory effect of sevoflurane in isolated human chorionic plate arterial rings. Quadruplicate ex vivo human chorionic plate arterial rings were used in all studies. Series 1 and 2 examined the role of the K+ channel in sevoflurane-mediated vasodilation. Separate experiments examined whether tetraethylammonium, which blocks large conductance calcium activated K+ (KCa++) channels (Series 1A+B) or glibenclamide, which blocks the ATP sensitive K+ (KATP) channel (Series 2), modulated sevoflurane-mediated vasodilation. Series 3 - 5 examined the role of the Ca++ channel in sevoflurane induced vasodilation. Separate experiments examined whether verapamil, which blocks the sarcolemmal voltage-operated Ca++ channel (Series 3), SK&F 96365 an inhibitor of sarcolemmal voltage-independent Ca++ channels (Series 4A+B), or ryanodine an inhibitor of the sarcoplasmic reticulum Ca++ channel (Series 5A+B), modulated sevoflurane-mediated vasodilation. Sevoflurane produced dose dependent vasodilatation of chorionic plate arterial rings in all studies. Prior blockade of the KCa++ and KATP channels augmented the vasodilator effects of sevoflurane. Furthermore, exposure of rings to sevoflurane in advance of TEA occluded the effects of TEA. Taken together, these findings suggest that sevoflurane blocks K+ channels. Blockade of the voltage-operated Ca++channels inhibited the vasodilator effects of sevoflurane. In contrast, blockade of the voltage-independent and sarcoplasmic reticulum Ca++channels did not alter sevoflurane vasodilation. Sevoflurane appears to block chorionic arterial KCa++ and KATP channels. Sevoflurane also blocks voltage-operated calcium channels, and exerts a net vasodilatory effect in the in vitro feto-placental circulation.
PMID:19515255 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702293 Jarman J et al; BMC Anesthesiol 9: 4 (2009)
Administration of sevoflurane at the onset of reperfusion has been confirmed to provide a cerebral protection. However, little is known about the mechanism. In this study, /investigators/ tested the hypothesis that sevoflurane postconditioning induces neuroprotection through the up-regulation hypoxia inducible factor-1 alpha (HIF-1 alpha) and heme oxygenase-1 (HO-1) involving phosphatidylinositol-3-kinase (PI3K)/Akt pathway. In the first experiment, male Sprague-Dawley rats were subjected to focal cerebral ischemia. Postconditioning was performed by exposure to 2.5% sevoflurane immediately at the onset of reperfusion. The mRNA and protein expression of HIF-1 alpha and its target gene, HO-1, intact neurons and the activity of caspase-3 was evaluated at 6, 24 and 72 hr after reperfusion. In the second experiment, ... the relationship between PI3K/Akt pathway and the expression of HIF-1alpha and HO-1 in the neuroprotection induced by sevoflurane /was investigated/. Cerebral infarct volume, apoptotic neuron and the expression of HIF-1alpha, HO-1 and p-Akt were evaluated at 24hr after reperfusion. Compared with the control group, sevoflurane postconditiong significantly ameliorated neuronal injury, up-regulated mRNA and protein levels of HIF-1 alpha and HO-1, inhibited the activity of caspase-3, and decreased the number of TUNEL-positive cells and infarct sizes. However, the selective PI3K inhibitor, wortmannin not only partly eliminated the neuroprotection of sevoflurane as shown by reducing infarct size and apoptotic neuronal cells, but also reversed the elevation of HIF-1 alpha, HO-1 and p-Akt expression in the ischemic penumbra induced by sevoflurane. Therefore, /the/ data demonstrates that the cerebral protection from sevoflurane postconditioning is partly mediated by PI3K/Akt pathway via the up-regulation of HIF-1 alpha and HO-1.
PMID:22580326 Ye Z et al; Brain Res 1463: 63-74 (2012)
Sevoflurane is widely used for anesthesia, and is commonly used together with opioids in clinical practice. However, the effects of sevoflurane on mu-opioid receptor (uOR) functions is still unclear. In this study, the effects of sevoflurane on uOR functions were analyzed by using Xenopus oocytes expressing a uOR fused to chimeric G alpha protein G(qi5) (uOR-G(qi5)). Sevoflurane by itself did not elicit any currents in oocytes expressing uOR-G(qi5), whereas sevoflurane inhibited the [D-Ala(2),N-Me-Phe(4),Gly(5)-ol]-enkephalin (DAMGO)-induced Cl(-) currents at clinically used concentrations. Sevoflurane did not affect the Cl(-) currents induced by AlF(4)(-), which directly led to activation of G proteins. The inhibitory effects of sevoflurane on the DAMGO-induced currents were not observed in oocytes pretreated with the protein kinase C (PKC) inhibitor GF109203X. These findings suggest that sevoflurane would inhibit uOR function. Further, the mechanism of inhibition by sevoflurane would be mediated by PKC.
PMID:21912198 Minami K et al; Pharmacology 88 (3-4): 127-32 (2011)
For more Mechanism of Action (Complete) data for Sevoflurane (9 total), please visit the HSDB record page.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38857
Submission : 2023-10-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11132
Submission : 1994-10-05
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24903
Submission : 2011-04-29
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2016-11-04
Pay. Date : 2016-08-15
DMF Number : 30570
Submission : 2016-09-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-12-09
Pay. Date : 2019-09-27
DMF Number : 22740
Submission : 2009-04-20
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19091
Submission : 2005-12-20
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2016-297 - Rev 03
Status : Valid
Issue Date : 2024-08-21
Type : Chemical
Substance Number : 2269

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2012-234 - Rev 00
Status : Valid
Issue Date : 2018-06-15
Type : Chemical
Substance Number : 2269

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Registration Number : 217MF10538
Registrant's Address : 5253 Okiube, Ube City, Yamaguchi Prefecture
Initial Date of Registration : 2005-09-09
Latest Date of Registration : 2010-02-23

Registration Number : 231MF10009
Registrant's Address : No. 7, Kunlunshan Road, Economic and Technological Development Zone, Lianyungang, Jia...
Initial Date of Registration : 2019-01-10
Latest Date of Registration : 2019-01-10

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Toru Corporation
Registration Date : 2021-01-21
Registration Number : 20210121-211-J-647
Manufacturer Name : Jiangsu Hengrui Pharmaceutic...
Manufacturer Address : Jinqiao Road, Dapu Industrial Park, Economic & Technological Development Zone Lianyun...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]VMF Number : 5512
Submission : 1994-08-01
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
About the Company : Supriya Lifescience Ltd. specializes in API manufacturing, focusing on therapeutic segments like antihistamines, anti-allergic drugs, vitamins, anaesthetics and anti-asthmatics. Su...
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
About the Company : Hebei AiYoung Pharmaceutical Technology Co., Ltd. is a professional company mainly engaged in chemical APIs and intermediates, including R & D, production, import and export. The c...

About the Company : Lunan Pharmaceutical Group is an integrated pharmaceutical group setting production, scientific research and sale of traditional Chinese medicines, chemicals, biological products i...

About the Company : Established in 2002, Tecoland represents selected cGMP manufacturers with proven capabilities in organic synthesis, fermentation production as well as process and method developmen...

About the Company : Zhuhai Rundu Pharmaceutical Co., Ltd. is a modern scientific and technological pharmaceutical enterprise integrating drug research and development, production, and sales. It is loc...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The Phase II SBIR funding will be used to develop a formulation of VPX638 (sevoflurane) and to further characterize the anti-inflammatory and analgesic profile in animal disease models.
Lead Product(s): Sevoflurane
Therapeutic Area: Trauma (Emergency, Injury, Surgery) Brand Name: VPX638
Study Phase: Phase IIProduct Type: Small molecule
Sponsor: National Institute of Arthritis and Musculoskeletal and Skin Diseases
Deal Size: $1.5 million Upfront Cash: Undisclosed
Deal Type: Funding November 11, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sevoflurane
Therapeutic Area : Trauma (Emergency, Injury, Surgery)
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : National Institute of Arthritis and Musculoskeletal and Skin Diseases
Deal Size : $1.5 million
Deal Type : Funding
Details : The Phase II SBIR funding will be used to develop a formulation of VPX638 (sevoflurane) and to further characterize the anti-inflammatory and analgesic profile in animal disease models.
Product Name : VPX638
Product Type : Small molecule
Upfront Cash : Undisclosed
November 11, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 250ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 100ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 50ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ULTANE
Dosage Form : LIQUID;INHALATION
Dosage Strength : 100%
Packaging :
Approval Date : 1995-06-07
Application Number : 20478
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Sevoflurane
Dosage Form : INHALATION VAPOR, LIQUID
Dosage Strength : -
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Sevorane
Dosage Form : Sevoflurane 100% 250Ml 1 Units Inhalation Use
Dosage Strength : 1 Bottle 250 ml gas inhal quik
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Sevorane swung a lot
Dosage Form : Fluid to inhalasjonsdamp
Dosage Strength :
Packaging : Bottle of plastic
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Canada
Brand Name : SEVORANE AF
Dosage Form : LIQUID
Dosage Strength : 99.97%
Packaging : 250 ML
Approval Date :
Application Number : 2172763
Regulatory Info :
Registration Country : Canada
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Ultane
Dosage Form : SLN
Dosage Strength : 100ml
Packaging : 250X1ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Canada
Brand Name : SEVOFLURANE
Dosage Form : LIQUID
Dosage Strength : 99.97%
Packaging : 250ML
Approval Date :
Application Number : 2265974
Regulatory Info :
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 250ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Inhaler solution
Dosage Strength : 250ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 100ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Inhaler solution
Dosage Strength : 100ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Inhaler solution
Dosage Strength : 50ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Packaging :
Regulatory Info :
Dosage : Inhaler solution
Dosage Strength : 50ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Brazil
Brand Name : SEVOCRIS
Dosage Form : INJECTION
Dosage Strength : 100%
Packaging : BOTTLE X 100ML
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Brazil

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : BOTTLE X 100ML
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 100%
Brand Name : SEVOCRIS
Approval Date :
Application Number :
Registration Country : Brazil

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Brazil
Brand Name : SEVOCRIS
Dosage Form : INJECTION
Dosage Strength : 100%
Packaging : BOTTLE X25OML
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Brazil

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : BOTTLE X25OML
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 100%
Brand Name : SEVOCRIS
Approval Date :
Application Number :
Registration Country : Brazil

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Korea
Brand Name : SevofranSolution
Dosage Form : SOLUTION
Dosage Strength : 250ML
Packaging : 250mL/BTL
Approval Date :
Application Number : 77867
Regulatory Info : Generic
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 250mL/BTL
Regulatory Info : Generic
Dosage : SOLUTION
Dosage Strength : 250ML
Brand Name : SevofranSolution
Approval Date :
Application Number : 77867
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : MA- US FDA, Germany BfArm, Spa...
Registration Country : China
Brand Name :
Dosage Form : Inhalation
Dosage Strength : 250ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : MA- US FDA, Germany BfArm, Spa...
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : MA- US FDA, Germany BfArm, Spa...
Dosage : Inhalation
Dosage Strength : 250ML
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Main Therapeutic Indication : Anaesthetic
Currency : USD
2017 Revenue in Millions : 410
2016 Revenue in Millions : 428
Growth (%) : -4
Main Therapeutic Indication : Anesthetics
Currency : USD
2016 Revenue in Millions : 428
2015 Revenue in Millions : 474
Growth (%) : -10
Main Therapeutic Indication : Others
Currency : USD
2015 Revenue in Millions : 550
2014 Revenue in Millions : 474
Growth (%) : -14%
Main Therapeutic Indication : Central Nervous System And Anesthes...
Currency : USD
2018 Revenue in Millions : 391
2017 Revenue in Millions : 410
Growth (%) : -5%
Main Therapeutic Indication : CNS & Anesthesia
Currency : USD
2019 Revenue in Millions : 348
2018 Revenue in Millions : 391
Growth (%) : -11
Main Therapeutic Indication : Others
Currency : USD
2014 Revenue in Millions : -3.20%
2013 Revenue in Millions :
Growth (%) :
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
19 Feb 2025
Reply
30 Oct 2024
Reply
10 Aug 2024
Reply
31 Jan 2024
Reply
25 Oct 2023
Reply
21 Jun 2022
Reply
02 Sep 2021
Reply
08 Feb 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Reply
18 Dec 2023
Reply
11 Jul 2023
Reply
25 May 2023
Reply
19 Jan 2021
Reply
28 Aug 2020
Reply
06 Jan 2020
Reply
06 Feb 2019
Reply
07 Feb 2018
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

CAS Number : 28523-86-6
Quantity Per Vial : 1 mL
Sale Unit : 1
Order Code : Y0001046
Batch No : 2
Price (€) : 79
Storage : +5°C ± 3°C

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]CAS Number : 28523-86-6
Quantity Per Vial :
Price ($) : 230
Catalog Number : 1612540
Current Lot : R071C0
Previous Lot : H1K268 (30-NOV-2017)
NDC Code :

Sevoflurane Related Compound A (0.2 mL) (1,1,...
CAS Number : 58109-34-5
Quantity Per Vial :
Price ($) : 805
Catalog Number : 1612550
Current Lot : R056M0
Previous Lot : R014Q0 (31-MAY-2017)
NDC Code :

Sevoflurane Related Compound B (0.2 mL) (1,1,...
CAS Number : 13171-18-1
Quantity Per Vial :
Price ($) : 805
Catalog Number : 1612572
Current Lot : R06670
Previous Lot : G0J231 (31-MAY-2018)
NDC Code :

Sevoflurane Related Compound C (0.2 mL) (1,1,...
CAS Number : 920-66-1
Quantity Per Vial :
Price ($) : 805
Catalog Number : 1612594
Current Lot :
Previous Lot : G0J253 (30-SEP-2018)
NDC Code :

Monograph in Japanese Pharmacopoeia : Sevofluran...
Package Size : 18 mL
Price (¥) : 52,457
Storage Temprature °C : 8°C
Assay Test : I/ IR A/ GC

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Sevoflurane Related Compound A (0.2 mL) (1,1,...
CAS Number : 58109-34-5
Quantity Per Vial : 0.2
Sale Unit : ml
Price : $835.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1612550 / R056M0

CAS Number : 28523-86-6
Quantity Per Vial : 1
Sale Unit : ml
Price : $245.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1612540 / R12620

Sevoflurane Related Compound B (0.2 mL) (1,1,...
CAS Number : 13171-18-1
Quantity Per Vial : 0.2
Sale Unit : ml
Price : $835.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1612572 / R06670

Sevoflurane Related Compound C (0.2\"mL) (1,1...
CAS Number : 920-66-1
Quantity Per Vial : 0.2
Sale Unit : ml
Price : $835.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1612594 / R077T0

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
49
PharmaCompass offers a list of Sevoflurane API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sevoflurane manufacturer or Sevoflurane supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sevoflurane manufacturer or Sevoflurane supplier.
PharmaCompass also assists you with knowing the Sevoflurane API Price utilized in the formulation of products. Sevoflurane API Price is not always fixed or binding as the Sevoflurane Price is obtained through a variety of data sources. The Sevoflurane Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sevoflurane manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sevoflurane, including repackagers and relabelers. The FDA regulates Sevoflurane manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sevoflurane API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sevoflurane manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sevoflurane supplier is an individual or a company that provides Sevoflurane active pharmaceutical ingredient (API) or Sevoflurane finished formulations upon request. The Sevoflurane suppliers may include Sevoflurane API manufacturers, exporters, distributors and traders.
click here to find a list of Sevoflurane suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sevoflurane DMF (Drug Master File) is a document detailing the whole manufacturing process of Sevoflurane active pharmaceutical ingredient (API) in detail. Different forms of Sevoflurane DMFs exist exist since differing nations have different regulations, such as Sevoflurane USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Sevoflurane DMF submitted to regulatory agencies in the US is known as a USDMF. Sevoflurane USDMF includes data on Sevoflurane's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Sevoflurane USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Sevoflurane suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Sevoflurane Drug Master File in Japan (Sevoflurane JDMF) empowers Sevoflurane API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Sevoflurane JDMF during the approval evaluation for pharmaceutical products. At the time of Sevoflurane JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Sevoflurane suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Sevoflurane Drug Master File in Korea (Sevoflurane KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Sevoflurane. The MFDS reviews the Sevoflurane KDMF as part of the drug registration process and uses the information provided in the Sevoflurane KDMF to evaluate the safety and efficacy of the drug.
After submitting a Sevoflurane KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Sevoflurane API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Sevoflurane suppliers with KDMF on PharmaCompass.
A Sevoflurane CEP of the European Pharmacopoeia monograph is often referred to as a Sevoflurane Certificate of Suitability (COS). The purpose of a Sevoflurane CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Sevoflurane EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Sevoflurane to their clients by showing that a Sevoflurane CEP has been issued for it. The manufacturer submits a Sevoflurane CEP (COS) as part of the market authorization procedure, and it takes on the role of a Sevoflurane CEP holder for the record. Additionally, the data presented in the Sevoflurane CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Sevoflurane DMF.
A Sevoflurane CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Sevoflurane CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Sevoflurane suppliers with CEP (COS) on PharmaCompass.
Sevoflurane Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sevoflurane GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sevoflurane GMP manufacturer or Sevoflurane GMP API supplier for your needs.
A Sevoflurane CoA (Certificate of Analysis) is a formal document that attests to Sevoflurane's compliance with Sevoflurane specifications and serves as a tool for batch-level quality control.
Sevoflurane CoA mostly includes findings from lab analyses of a specific batch. For each Sevoflurane CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sevoflurane may be tested according to a variety of international standards, such as European Pharmacopoeia (Sevoflurane EP), Sevoflurane JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sevoflurane USP).
